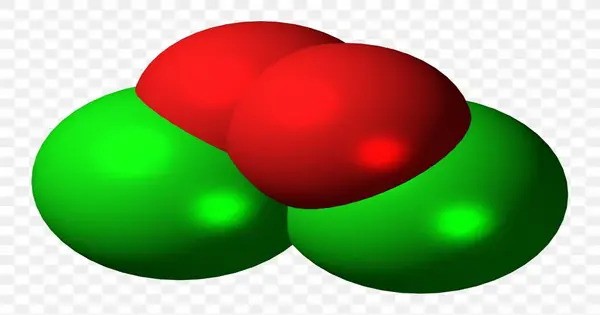
Chlorine peroxide (also known as dichlorine dioxide or ClO dimer) is a molecular compound with formula ClOOCl. It is typically unstable and highly reactive, meaning it’s not commonly found in everyday use. Chemically, it is a dimer of the chlorine monoxide radical (ClO·). It is important in the formation of the ozone hole.
Chlorine peroxide catalytically converts ozone into oxygen when it is irradiated by ultraviolet light. It has been researched for use in certain industrial applications, such as bleaching or disinfection, due to its oxidizing properties.
Properties
Chlorine peroxide is typically a reddish-brown liquid, though it may also appear as a solid depending on temperature. It is highly unstable and can decompose explosively, especially under conditions of heat or shock. It is a strong oxidizing agent, reacting violently with organic compounds, reducing agents, and other substances. It is soluble in organic solvents but can decompose in the presence of moisture or water.
- Chemical formula: Cl2O2
- Molar mass: 102.905 g/mol
Production
Chlorine peroxide can be produced by laser or ultraviolet photolysis of the chlorine molecule with ozone. The lasers used to break up the chlorine molecule into atoms can be an excimer laser at 248, 308, or 352 nm wavelength. Difluorodichloromethane (CF2Cl2) can also act as a source of chlorine atoms for the formation of the peroxide. Microwave discharge can also break up chlorine molecules into atoms that react with ozone to make chlorine peroxide.
Cl2 + hν → 2Cl
Cl + O3 → O2 + ClO·
2ClO· + M → ClOOCl + M
ClOOCl + hν → Cl + ClO2
ClO2 + M → Cl + O2 + M
Occurrence
Chlorine peroxide is not commonly found in nature due to its instability. It is generally produced in laboratories under controlled conditions by reacting chlorine gas (Cl₂) with hydrogen peroxide (H₂O₂), though only in small amounts due to its explosive nature.
Chlorine peroxide is used primarily in chemical research and has potential applications in the synthesis of various chemicals and materials, though its practical use is limited by its volatility and difficulty in storage.