A chemical element is a substance that contains only one type of atom. If a substance contains more than one type of atom, it is a compound. An element can be a solid, liquid or gas. They are the fundamental materials of which all matter is composed. The smallest particle of such an element is an atom. Atoms are made up of protons, neutrons, and electrons.
The number of protons in the nucleus causes its electric charge. This fixes the number of electrons in its normal (unionized) state. The electrons in their atomic orbitals determine the atom’s various chemical properties. Atoms of the same element have the same atomic number or Z.
Elements are the basic building blocks for all types of substances. When they are combined with each other, they can form molecules. Each element is identified according to the number of protons it has in its atomic nucleus.
118 different chemical elements are known to modern chemistry. About 20 percent of them do not exist in nature and are known only because they have been synthetically prepared in the laboratory. 92 of these elements can be found in nature, and the others can only be made in laboratories. The human body is made up of 26 elements. The last natural element discovered was uranium, in 1789. The first man-made element was technetium, in 1937.
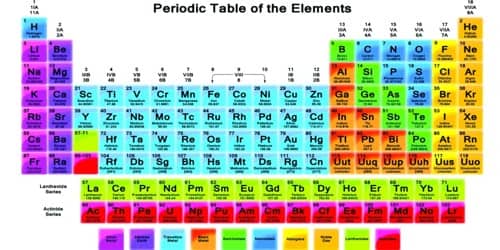
Chemical elements are commonly arranged in the periodic table. Where the elements are on the table tells us about their properties relative to the other elements.
Chemical symbols
Chemical elements are also given a unique chemical symbol. Chemical symbols are used all over the world. This means that no matter which language is spoken, there is no confusion about what the symbol means. Chemical symbols of elements come from their English or Latin names. For example, carbon has the chemical symbol ‘C’, and sodium has the chemical symbol ‘Na’, after the Latin natrium. Tungsten is called ‘W’ after its German name, wolfram. ‘Au’ is the symbol for gold and it comes from the Latin word for gold, aurum.
Compounds
Elements can join (react) to form pure compounds (such as water, salts, oxides, and organic compounds). In many cases, these compounds have a fixed composition and their own structure and properties. Elements can combine with one another to form a wide variety of more complex substances called compounds. The properties of the compound may be very different from the elements it is made from. Sodium is a metal that burns when put into water and chlorine is a poisonous gas. When they react together they make sodium chloride (salt) which is harmless and edible.
Mixtures
Some elements mix together in any proportion to form new structures. Mixtures differ from compounds in that they can be separated into their component parts by physical processes; for example, the simple process of evaporation separates water from the other compounds in seawater. Such new structures are not compounds. They are called mixtures or when the elements are metals, alloys.
Isotopes
Most elements in nature consist of atoms with different numbers of neutrons. An isotope is a form of an element with a certain number of neutrons. Some isotopes are radioactive and decay into other elements upon radiating an alpha or beta particle. For example, carbon has two stable, naturally occurring isotopes: carbon-12 (6 neutrons) and carbon-13 (7 neutrons). Carbon-14 (8 neutrons) is a naturally occurring radioactive isotope of carbon.
Classification
Elements can be classified based on physical states. At room temperature and pressure, most elements are solids, only 11 are gases and 2 are liquids.
Elements can also be classified into metals and non-metals. There are many more metals than non-metals.