Researchers at the Department of Energy’s Oak Ridge National Laboratory are researching battery technology to combat climate change in two ways: by increasing the usage of renewable energy and by capturing airborne CO2.
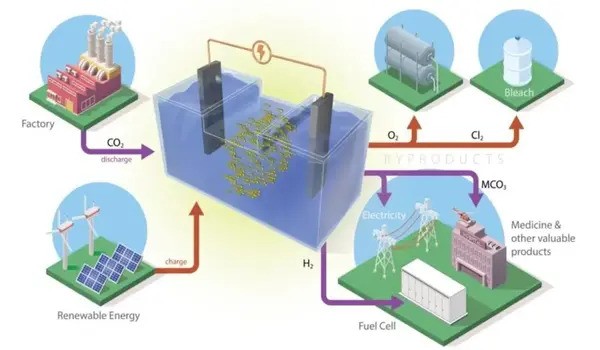
This type of battery stores renewable energy produced by solar panels or wind turbines. When wind and sunlight are not available, an electrochemical reaction is required, which, in the case of ORNL’s new battery formulation, collects carbon dioxide from industrial emissions and converts it to valuable chemicals.
ORNL researchers have developed and tested two distinct battery formulations that transform carbon dioxide gas (CO2) into a solid form that could be used in other products.
One of these novel battery types held its charge for 600 hours and could store up to 10 hours of electricity. Researchers also recognized, studied, and overcome the primary issue, a deactivation induced by chemical accumulation, which had been an impediment to the previous battery composition.
We are reporting for the first time that the deactivated cell can be reactivated. And we found the origin of the deactivation and activation. If you symmetrically charge-discharge the battery too long, it’s dead at one stage. If you use the protocol we established for our cell, the chance of failure is very slim.
Ruhul Amin
“The Transformation Energy Science and Technology, or TEST, initiative at ORNL is precisely the kind of effort needed to address climate change. We are excited that ORNL is investing in innovative ideas and approaches that can transform the way we think about storing energy beyond lithium-ion batteries and other conventional electrochemical energy storage systems,” said Ilias Belharouak, an ORNL Corporate Fellow and initiative director. “What a fantastic scenario: Using free electrons to store CO2 and converting it to revenue-generating products is a concept I never would have imagined 10 years back, but this is just a start.”
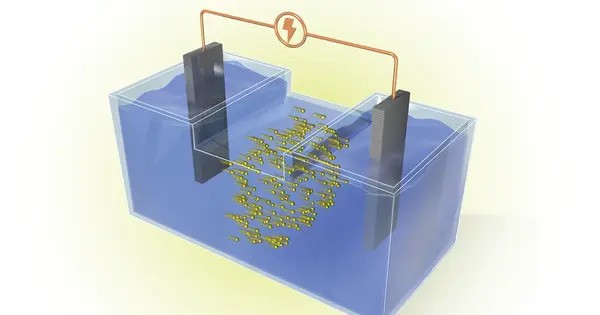
Batteries work by electrochemical reactions that transport ions between two electrodes via an electrolyte. Unlike cell phone or car batteries, grid energy storage batteries are not required to be portable or closed systems. This enabled ORNL researchers to design and test two types of batteries capable of converting CO2 from stationary, industrial sources.
For example, CO2 produced by a power plant may be fed through a tube into the liquid electrolyte, resulting in bubbles similar to those found in carbonated soft drinks. During battery operation, the gas bubbles solidify into a powder.
How it works
Each component of a battery might be composed of several elements or compounds. These options impact the battery’s operational lifetime, the amount of energy it can store, its size and weight, and how quickly it charges or consumes energy. One of the new ORNL battery compositions mixes CO2 and sodium from saltwater with a cheap iron-nickel catalyst. The second combines the gas and aluminum.
Each method employs abundant materials and a liquid electrolyte in the form of saltwater, which is occasionally combined with additional chemicals. Ruhul Amin, the principal researcher, stated that the batteries are safer than conventional technology since their electrodes are stable in water.
Very little CO2 battery research has been conducted. The previously-tried approach relies on a reversible metal-CO2 reaction that regenerates carbon dioxide, continuing to contribute greenhouse gases to the atmosphere. In addition, solid discharge products tend to clog the surface of the electrode, degrading the battery performance.
However, the CO2 batteries developed at ORNL do not release carbon dioxide. Instead, the carbonate byproduct dissolves in the liquid electrolyte. The byproduct either continuously enriches the liquid to enhance battery performance, or it can be filtered from the bottom of the container without interrupting battery operation. Battery design can even be tuned to create more of these byproducts for use by the pharmaceutical or cement industries. The only gases released are oxygen and hydrogen, which do not contribute to climate change and can even be captured to produce energy or fuel.
ORNL researchers used an almost completely new combination of materials for these CO2 batteries. The few similar previous designs worked for only short periods or incorporated expensive metals.
Pros, cons and challenges overcome
The sodium-carbon dioxide, or Na-CO2, battery was created first and had several difficulties. To function properly, the electrodes must be separated in wet and dry chambers by a solid ion conductor. The barrier inhibits the passage of ions, which slows battery action and decreases battery efficiency.
One key problem for this Na-CO2 battery is that over time, a layer accumulates on the electrode surface, causing the battery to deactivate. Amin’s research team examined the battery cell at various stages of operation and when it failed using highly advanced microscopes and X-ray techniques.
Studying how the film formed helped researchers understand how to break it down again. They were intrigued to realize the battery could be reactivated, or prevented from deactivating at all, simply through operational changes in the charge/discharge cycle. Uneven pulses of charging and discharging prevented film buildup on the electrode.
“We are reporting for the first time that the deactivated cell can be reactivated,” Amin said. “And we found the origin of the deactivation and activation. If you symmetrically charge-discharge the battery too long, it’s dead at one stage. If you use the protocol we established for our cell, the chance of failure is very slim.”
A second design for long-term storage
Next, researchers focused on the design of the aluminum-carbon dioxide, or Al-CO2, battery. The team experimented with various electrolyte solutions and three different synthesis processes to identify the best combination. The result was a battery which provides enough storage for more than 10 hours of electricity to be used later.
“That’s huge for long-duration storage,” Amin said. “This is the first Al-CO2 battery that could run with stability for a long time, which is the goal. Holding just a few hours of stored energy doesn’t help.”
Testing found that the ORNL battery could operate more than 600 hours without losing capacity, Amin said — far more than the only previously reported Al-CO2 battery, which was only tested for eight hours of cycling.
The cherry on top is that this battery absorbs nearly twice as much CO2 as the Na-CO2 battery. The system can be built to function in a single chamber, with both electrodes immersed in the same liquid solution, eliminating any barriers to ion transport.
Amin stated that the difficulty for the Al-CO2 battery is to bring it closer to scaling up. Nonetheless, the team will continue to rigorously investigate its features in order to increase its operational lifetime and trap CO2 more efficiently. To make the Na-CO2 battery competitive, the researchers will work on creating a very fine, dense, and mechanically stable ceramic membrane to divide the battery chambers.