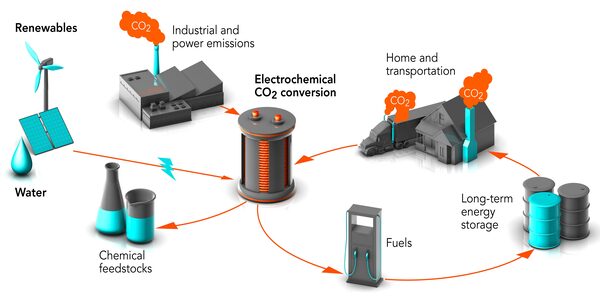
Researchers have successfully converted CO2 into methanol by shining sunlight on individual copper atoms put on a light-activated polymer, paving the way for the development of new green fuels.
An international team of researchers from the University of Nottingham’s School of Chemistry, the University of Birmingham, the University of Queensland, and the University of Ulm created a material composed of copper anchored in nanocrystalline carbon nitride. The copper atoms are nested within the nanocrystalline structure, allowing electrons to pass from carbon nitride to CO2, a critical step in the creation of methanol from CO2 under solar irradiation. The results were published in the Royal Society of Chemistry’s Sustainable Energy & Fuels Journal.
In photocatalysis, light is shone on a semiconductor material, which excites electrons and allows them to flow through the material to react with CO2 and water, producing a range of useful chemicals, including methanol, a green fuel. Despite recent advancements, this method lacks efficiency and selectivity.
We measured the current generated by light and used it as a criterion to judge the quality of the catalyst. Even without copper, the new form of carbon nitride is 44 times more active than traditional carbon nitride.
Tara LeMercier
Carbon dioxide is the most significant contributor to global warming. Although it is feasible to transform CO2 into valuable goods, most thermal methods rely on hydrogen derived from fossil fuels. It is critical to develop alternative technologies based on photo- and electrocatalysis that take advantage of renewable solar energy and the abundance of available water.
Dr Madasamy Thangamuthu, a research fellow in the School of Chemistry, University of Nottingham, who co-led the research team, said: “There is a large variety of different materials used in photocatalysis. It is important that the photocatalyst absorbs light and separates charge carriers with high efficiency. In our approach, we control the material at the nanoscale. We developed a new form of carbon nitride with crystalline nanoscale domains that allow efficient interaction with light as well as sufficient charge separation.”
The researchers devised a process of heating carbon nitride to the required degree of crystallinity, maximizing the functional properties of this material for photocatalysis. Using magnetron sputtering, they deposited atomic copper in a solventless process, allowing intimate contact between the semiconductor and metal atoms.
Tara LeMercier, a PhD student who carried out the experimental work at the University of Nottingham, School of Chemistry, said: “We measured the current generated by light and used it as a criterion to judge the quality of the catalyst. Even without copper, the new form of carbon nitride is 44 times more active than traditional carbon nitride. However, to our surprise, the addition of only 1 mg of copper per 1 g of carbon nitride quadrupled this efficiency. Most importantly the selectivity changed from methane, another greenhouse gas, to methanol, a valuable green fuel.”
According to Professor Andrei Khlobystov of the University of Nottingham’s School of Chemistry, carbon dioxide valorization is critical to meeting the UK’s net-zero target. It is critical to ensure the longevity of our catalyst materials for this key reaction. A significant advantage of the new catalyst is that it is composed of sustainable components such as carbon, nitrogen, and copper, all of which are abundant on our planet.
This invention is a huge step forward in our understanding of photocatalytic materials in CO2 conversion. It paves the way for the development of extremely selective and tuneable catalysts, with the desired product being dialed up via nanoscale control of the catalyst.
This research is supported by the EPSRC Programme Grant ‘Metal atoms on surfaces and interfaces (MASI) for a Sustainable Future’, which aims to develop catalytic materials for the conversion of three major chemicals – carbon dioxide, hydrogen, and ammonia – that are critical for the economy and the environment. MASI catalysts are created in an atom-efficient manner to assure long-term usage of chemical components while avoiding depletion of rare elements and producing the majority of the earth’s abundant elements, such as carbon and base metals.