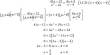Carbide bromides are mixed anion compounds that contain both bromide and carbide ions. These are chemical compounds in which carbide (carbon bound with a metal, usually a transition metal) is mixed with bromine. Many carbide bromides are cluster compounds with one, two, or more carbon atoms in a core, surrounded by a layer of metal atoms and enclosed in a shell of bromide ions. These ions can be distributed across clusters to form chains, double chains, or layers. These compounds are commonly classified as metal carbides with bromine atoms bonded to the carbide structure.
The majority of these carbide bromide compounds include rare earth elements. Because these elements have comparable properties, swapping them yields similar structures. R2CBr2 is a structure composed of layers of R6C clusters, each containing one carbon atom. Each layer has a bromide coating on top and bottom. R2CBr2 is quite similar, as it has layers of R6C2 clusters holding pairs of carbon atoms. This dicarbon is an ethenide (C24−), with a double bond.
Layers have bromide on both sides, thus van der Waals forces can only hold them together loosely. If these layers are aligned, a 1T-form with a tiny c measurement on the unit cell is obtained. In other compounds, the layers are not perfectly aligned, but they repeat after three layers, resulting in a 3R structure with a greater c unit cell height. When the layers align, the crystal system is trigonal. However, if the layers never completely align at any height, a monoclinic crystal forms. The C2 unit lies at an angle to the layers, reducing symmetry as compared to compounds containing single carbon atoms in the cluster.
CaC₂Br₂ is an example of a carbide bromide. This chemical contains calcium (Ca), carbide (C₂), and bromine (Br₂). These can have a variety of qualities and applications depending on the metal used and the conditions under which they are synthesized. They can be used in chemical reactions, as catalysts, or in materials research due to their unique chemical and physical properties.