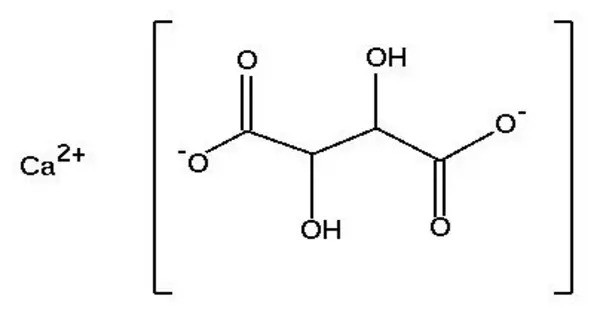
Calcium tartrate, exactly calcium L-tartrate, is a byproduct of the wine industry, prepared from wine fermentation dregs. It is the calcium salt of tartaric acid, which is a naturally occurring organic acid commonly found in grapes and in various fruits. It appears as a white, crystalline solid, and it is relatively insoluble in water.
It is the calcium salt of L-tartaric acid, an acid most commonly found in grapes. Its solubility decreases with lower temperature, which results in the forming of whitish (in red wine often reddish) crystalline clusters as it precipitates. As E number E354, it finds use as a food preservative and acidity regulator.
Properties
- Chemical formula: CaC4H4O6
- Molar mass: 190.16484 g/mol (anhydrous), 260.21 g/mol (tetrahydrate)
- Appearance: hygroscopic white powder, or colorless crystals
- Density: 1.817 g/cm3 (tetrahydrate)
- Melting point: tetrahydrate decomposes at 160 °C, anhydrous decomposes at 650 °C
- Solubility in water: 0.037 g/100 ml (0 °C) 0.2 g/100 ml (85 °C)
Occurrences
Natural Occurrence
Calcium tartrate is commonly found as a natural mineral, often referred to as “tartrate of lime”. It occurs in certain fruits, especially in grapes, where it is found as part of the sediment in wine-making (as potassium or calcium tartrate crystals). Tartar deposits in wine bottles are primarily composed of potassium bitartrate, but calcium tartrate can also be present, especially when the pH is higher.
In Plants and Fruits: It is found in the tissues of certain plants and fruits, particularly in grapes. It is deposited as crystals in the skins and stems of fruits, and also found in some other plants, where it can accumulate as a byproduct of metabolic processes.
In Wine-making: During wine fermentation, it can precipitate out as a byproduct, especially under cool storage conditions. It can form as crystals in wine bottles, commonly referred to as “wine diamonds”. To prevent its formation, wine is often chilled to encourage the crystallization process before bottling, a practice known as cold stabilization.
In the Human Body: It is not typically present in significant amounts in the human body, but trace amounts of tartaric acid salts can be found in some foods, particularly those containing tartaric acid like wine, grapes, or certain fruits.
Uses
- Winemaking: It is a byproduct in the winemaking process, where it can form crystals (known as “wine diamonds”) in wine during cold stabilization. This process is done to prevent the crystals from forming later in the bottle.
- Food Additive: It may be used in some food and beverage applications as a stabilizer or to enhance the texture of certain products.
- Medicinal: In some contexts, calcium tartrate is used in supplements to provide calcium, although it is not as commonly used as other forms like calcium carbonate or calcium citrate.
Formation
Calcium tartrate can form when calcium ions (from calcium salts) react with tartaric acid or its derivatives. It is also a common component of urinary stones in humans and animals, specifically in cases where there is an excess of calcium or tartaric acid in the urine.