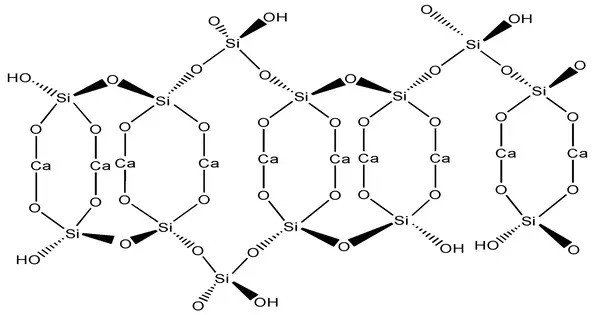
Calcium silicate hydrates (CSH or C-S-H) are the main products of the hydration of Portland cement and are primarily responsible for the strength of cement-based materials. They form when cement reacts with water (hydration), producing a gel-like, amorphous or poorly crystalline structure. They are the main binding phase (the “glue”) in most concrete. It fills the space between cement grains, reducing porosity and enhancing mechanical properties.
C-S-H has a high surface area, contributing to its ability to bind materials and retain water. Despite being poorly ordered, its layered structure resembles natural minerals like tobermorite. Only well defined and rare natural crystalline minerals can be abbreviated as CSH while extremely variable and poorly ordered phases without well defined stoichiometry, as it is commonly observed in hardened cement paste (HCP), are denoted C-S-H.
C-S-H plays a crucial role in determining the long-term performance of concrete, including resistance to chemical attack, creep, and shrinkage.
Properties
- Color: White to grey
- Texture: Gel-like to powdery
- Surface area: High specific surface area (~100–300 m²/g)
- Porosity: High gel porosity; critical for water retention and ion diffusion
Preparation
When water is added to cement, each of the compounds undergoes hydration and contributes to the final state of the concrete. Only calcium silicates contribute to the strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, only contributes to late strength. Calcium silicate hydrate (also shown as C-S-H) is a result of the reaction between the silicate phases of Portland cement and water. This reaction typically is expressed as:
2 Ca3SiO5 + 7 H2O → 3 CaO · 2 SiO2 · 4 H2O + 3 Ca(OH)2 + 173.6 kJ
also written in cement chemist notation, (CCN) as:
2 C3S + 7 H → C3S2H4 + 3 CH + heat
or, tricalcium silicate + water → calcium silicate hydrate + calcium hydroxide + heat
The stoichiometry of C-S-H in cement paste is variable and the state of chemically and physically bound water in its structure is not transparent, which is why “-” is used between C, S, and H.
Occurrences
(a) In Cement and Concrete
- Primary hydration product of alite (C₃S) and belite (C₂S) phases in Portland cement.
- Accounts for 50–60% of the solid volume in hydrated cement paste.
(b) In Natural Environments (Rare)
Rarely found in nature due to its metastable and amorphous nature. Natural analogues include:
- Tobermorite: A natural crystalline calcium silicate hydrate found in hydrothermally altered rocks.
- Jennite: Another mineral phase with a structure similar to synthetic C-S-H.
- Found in altered volcanic rocks, skarns, and hydrothermal veins.
(c) In Industrial and Synthetic Systems
- Synthesized in geopolymers and low-carbon cements.
- Engineered for CO₂ sequestration and nuclear waste encapsulation.