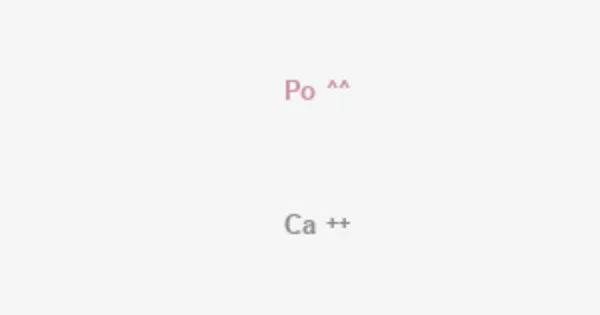
Calcium polonide (CaPo) is an intermetallic compound composed of calcium and polonium. Rather than being found in nature, the compound is wholly synthetic and difficult to research due to polonium’s high vapor pressure, radioactivity, and easy oxidation in air. Polonium is a highly radioactive material, and its most stable isotope, polonium-210 (Po-210), is well known for its severe radioactivity.
Properties
- Radioactivity: It is a radioactive compound due to the presence of polonium. Polonium isotopes, such as Po-210, are highly radioactive, emitting alpha particles.
- Appearance: It is typically found in solid form. It may appear as a grayish or metallic-looking material.
- Stability: It is not stable and undergoes radioactive decay. Polonium isotopes, including Po-210, have relatively short half-lives, which means they decay into other elements relatively quickly.
Production
Calcium polonide can be produced by combining calcium metal with polonium metal. However, the production and handling of polonium and its compounds require specialized equipment and strict safety measures.
Structure
It crystallizes in the cubic rock crystal salt structure at atmospheric pressure. The structure is projected to transition to a caesium chloride-type crystal structure at a high pressure of roughly 16.7 GPa.
Electronic properties
Calcium polonide is projected to be a semiconductor based on theoretical predictions. Because of the tremendous radioactivity of polonium-210, which makes it dangerous to handle, this chemical is not usually encountered or investigated. Polonium-210 decays into stable lead-206 (Pb-206) with a half-life of roughly 138 days, producing alpha particles (helium nuclei). Because of its radioactivity, polonium-210 is an extremely dangerous chemical that must be handled with extreme caution.
Uses
Calcium polonide has few practical applications due to its radioactivity. Some polonium isotopes, on the other hand, are used in industrial applications such as static eliminators and nuclear batteries. Small amounts of polonium are frequently used in these applications.
Health Hazards
Because of its radioactivity, calcium polonide, like all polonium-containing substances, poses serious health risks. Even minute doses of polonium inhalation or ingestion can be lethal. It is critical to handle polonium compounds with utmost caution and to adhere to strict safety measures.