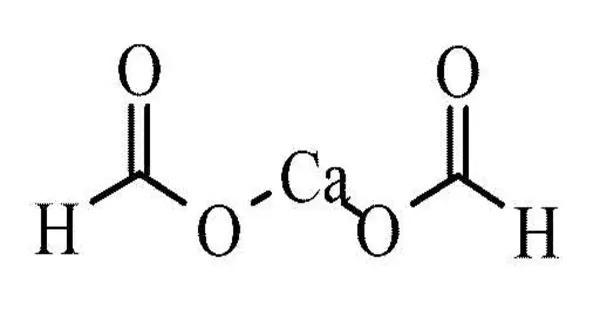
Calcium formate is the calcium salt of formic acid. It is also known as E238. Under this E number it is used as an animal feed preservative within EU, but not in foods intended for people. It’s a calcium salt of formic acid. It is commonly used in a variety of applications, including as a concrete additive, a preservative in animal feed, and in the production of certain chemicals.
Calcium formate is stable at room temperature, is flammable and forms orthorhombic crystals. The mineral form is very rare and called formicaite, and is known from a few boron deposits.
Properties
Calcium formate is typically a white, odorless, crystalline powder. It is moderately soluble in water and its solubility increases with temperature. It is stable under normal conditions but can decompose at higher temperatures to release carbon monoxide and carbon dioxide. It tends to absorb moisture from the air.
- Chemical formula: Ca(HCO2)2
- Molar mass: 130.113 g/mol
- Appearance: white-to-yellow crystals or crystalline powder
- Odor: smells slightly like acetic acid
- Density: 2.02 g/cm3
- Melting point: decomposes at 300 °C
- Solubility in water: 16.1 g/100 g (0 °C), 18.4 g/100 g (100 °C)
- Solubility: insoluble in ethanol, methanol: 0.27 g/100 g (15 °C), 0.23 g/100 g (66 °C)
Production
Calcium formate is formed as a co-product during trimethylolpropane production. Hydrated lime (calcium hydroxide) is used as the source of calcium. Butyraldehyde and formaldehyde react in a water solution in the presence of a basic catalyst, forming an unstable intermediate product, dimethylol butyraldehyde (DIMBA). DIMBA reacts further with formaldehyde to give trimethylolpropane and calcium formate. Calcium formate is separated from the solution, heat treated to remove formaldehyde and then dried.
Calcium formate can also be made from calcium hydroxide and carbon monoxide at high pressure and temperature – e.g., at 180 °C and 35 atm. It may also be made from calcium chloride and formic acid.
Occurrences
- Natural Occurrence: Calcium formate is not commonly found in nature as a mineral, but it can be produced synthetically.
- Manufacturing: It is primarily synthesized in industries by reacting calcium hydroxide (lime) with formic acid or sodium formate.
Use in Agriculture
Calcium formate is sometimes used as a feed additive for livestock because it can promote growth and improve feed utilization.
Safety
Pure calcium formate powder irritates eyes severely, but causes no skin irritation. Powder inhalation can be dangerous. The compound has a stinging taste. Ingesting liquids with high calcium formate concentrations cause severe gastrointestinal lesions.