Caesium telluride or Caesium telluridocaesium is an inorganic salt with a chemical formula Cs2Te. Caesium telluride is used to make photo cathodes. t is a type of alkali metal telluride, and its properties are of interest in various fields, such as materials science and electronics. It’s a semiconductor material with interesting properties, especially in the context of photoelectric and thermionic applications.
Caesium telluride is the photoemissive material used in many laser-driven radio frequency (RF) electron guns like in the TESLA Test Facility (TTF). It is also used as a photoemissive material, meaning it can emit electrons when exposed to light. This makes it useful in applications like photomultipliers and other devices that rely on the photoelectric effect.
Properties
- Chemical formula: Cs2Te
- Molar mass: 393.4
- Appearance: Crystalline solid
- Boiling point: 395.717128
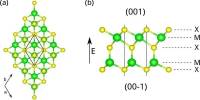
- Crystal Structure: It crystallizes in a cubic structure, specifically in the rock salt (NaCl) crystal structure. This is similar to many other alkali metal halides and tellurides.
- Solubility: It is insoluble in water but may dissolve in some acids due to the reactivity of caesium with acids.
- Electrical Conductivity: It has semiconductor properties. It exhibits electrical conductivity, which can change under different environmental conditions or with doping, making it useful in certain semiconductor applications.
- Thermal Properties: It has a relatively high melting point, around 1,050°C (1,922°F), which is typical for many tellurides. It also has a relatively high thermal stability.
Occurrences
Caesium telluride is not commonly found as a natural mineral, and it is not something that occurs in large quantities in nature. However, it can be synthesized in laboratories. Here are some occurrences and relevant uses:
Synthesis
Caesium telluride is typically synthesized in laboratories by directly combining caesium and tellurium in a controlled environment, often under high temperatures.
In Industry
It has applications in semiconductor technology and photovoltaics (especially in research related to advanced materials for solar cells or radiation detectors). It can also be used in thermoelectric devices and photoelectric applications, where its semiconductor properties are utilized.
Applications
- Solar Cells: As a semiconductor, it could theoretically be used in the production of solar cells.
- Thermionic Emission: Caesium telluride is also explored in thermionic emission technologies, where it may function in converting heat into electrical energy.
- Photoelectron Devices: Due to its ability to emit electrons when exposed to light, it’s used in scientific instruments requiring photoemission.