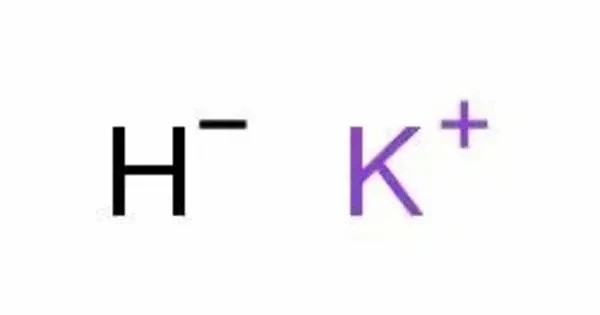
Caesium hydride or cesium hydride is an inorganic compound of caesium and hydrogen with the chemical formula CsH. It is an alkali metal hydride. It is a highly reactive compound, synthesized through the reaction of caesium metal and hydrogen gas. It was the first substance to be created by light-induced particle formation in metal vapor, and showed promise in early studies of an ion propulsion system using caesium. It is the most reactive stable alkaline metal hydride of all. It is a powerful superbase and reacts with water extremely vigorously.
It is primarily used in research and chemical synthesis as a strong base and hydride donor. The compound does not naturally occur and is produced in controlled laboratory conditions. Its strong reactivity, especially with water, means it must be handled carefully. It is very difficult to make caesium hydride in a pure form. Caesium hydride can be produced by heating caesium carbonate and metallic magnesium in hydrogen at 580 to 620 °C.
Properties
- Chemical formula: CsH
- Molar mass: 133.91339 g/mol
- Appearance: White or colorless crystals or powder
- Density: 3.42 g/cm3
- Melting point: ~170 °C (decomposes)
Caesium hydride is synthetically produced and not typically found in nature. It can be prepared by reacting caesium metal with hydrogen gas under controlled conditions. In its pure form, CsH is usually used in laboratory settings.
Synthesis
Caesium hydride is generally produced by reacting caesium metal (Cs) with hydrogen gas (H₂) at high temperatures:
2Cs + H2 → 2CsH
This reaction is often carried out in an inert atmosphere (such as argon) to prevent the caesium from reacting with oxygen.
The caesium nucleus in CsH can be hyperpolarized through interactions with an optically pumped caesium vapor in a process known as spin-exchange optical pumping (SEOP). SEOP can increase the nuclear magnetic resonance (NMR) signal of caesium nucleus by an order of magnitude.
Use in Chemistry
- It is used as a strong base in organic and inorganic synthesis.
- It is useful in the preparation of organometallic compounds, particularly for reactions where a powerful hydride donor is required.
- In some cases, CsH is used in the removal of protons from certain compounds in research or industrial applications.