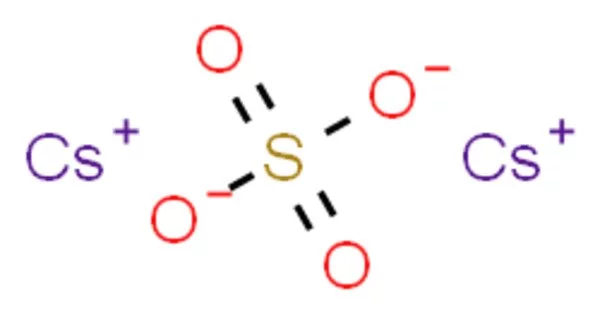
Caesium bisulfate or cesium hydrogen sulfate is an inorganic compound with the formula CsHSO4. The caesium salt of bisulfate, it is a colorless solid obtained by combining Cs2SO4 and H2SO4. It is a salt formed by the combination of caesium hydroxide (CsOH) and sulfuric acid (H₂SO₄). Like other bisulfates, it is an acidic salt, meaning it can release protons (H⁺) in aqueous solutions.
This compound is highly soluble in water and can be used in various chemical processes, including in the production of caesium-based compounds and as a catalyst in some reactions. It’s also of interest in some analytical and industrial applications.
Properties
Above 141 °C, CsHSO4 is a superionic conductor. The rapid ionic conductivity arise especially in the range of these temperatures due to the high activity of protons. Based on the results of X-ray crystallography, the structure consists of tetrahedral sulfate centers that bridge caesium ions. The proton is associated with the oxygen on sulfate.
- Chemical formula: CsHO4S
- Molar mass: 229.97 g·mol−1
- Appearance: White crystalline solid
- Solubility: It is soluble in water and dissolves to form a solution that is acidic.
- Acidity: The compound is acidic due to the presence of the bisulfate (HSO₄⁻) ion, which can dissociate into hydrogen ions (H⁺) in aqueous solutions.
- Melting Point: It has a high melting point (around 320°C), reflecting its stability at high temperatures.
Occurrences
Caesium bisulfate is typically not found naturally in significant quantities, as cesium is a rare element in Earth’s crust. However, cesium can be extracted from various minerals like pollucite (CsAlSi₂O₆·H₂O), which can be found in places like Canada and Zimbabwe. In practice, cesium bisulfate is synthesized in the lab or industrial settings rather than occurring naturally.
Potential applications
The maximum conductivity of pure CsHSO4 is 10 mS/cm, which is too low for practical applications. In composites with SiO2, TiO2, and Al2O3), the proton conductivity below the phase transition temperature is enhanced by a few orders of magnitude.
Unlike hydrated protonic conductors, the absence of water in CsHSO4 provides thermal and electrochemical stability. Electromotive force (EMF) measurements in a humidified oxygen concentration cell verified the high ionic nature of CsHSO4 in its superprotonic phase.