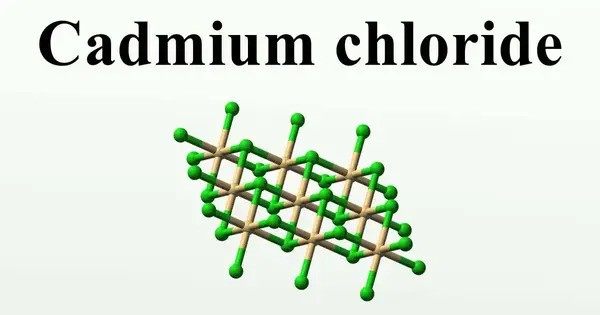
Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. It is an inorganic compound that appears as a white or yellowish solid, and it is highly soluble in water. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol.
Cadmium compounds, including cadmium chloride, are toxic and can pose serious health risks, such as kidney damage and respiratory issues, upon exposure. Environmental regulations are in place to limit cadmium’s release into ecosystems, given its potential for bioaccumulation and adverse effects on wildlife.
Properties
- Chemical formula: CdCl2
- Molar mass: 183.31 g·mol−1
- Appearance: White solid, hygroscopic
- Odor: Odorless
- Density: 4.047 g/cm3 (anhydrous), 3.26 g/cm3 (monohydrate)
- Melting point: 568 °C (1,054 °F; 841 K)
- Boiling point: 964 °C (1,767 °F; 1,237 K)
Occurrences
- Natural Sources: Cadmium chloride can occur naturally in mineral deposits, often associated with zinc ores. It can also form as a byproduct of other mining activities.
- Industrial Production: It is typically produced from the reaction of cadmium with hydrochloric acid or as a byproduct of zinc refining.
- Biological Context: While not essential for biological functions, cadmium can accumulate in living organisms, often leading to toxic effects.
Applications
It is used in various applications, including electroplating, the manufacture of batteries, as a stabilizer in plastics, and in some types of photography.
Safety Precautions
When handling cadmium chloride, it’s crucial to wear appropriate personal protective equipment (PPE), including gloves and goggles, and to work in a well-ventilated area or fume hood to minimize exposure.