The University of Michigan identified a way to keep perovskite semiconductors from degrading too quickly, which could help enable solar cells that are two to four times cheaper than today’s thin-film panels.
Perovskites can also be mixed with silicon-based semiconductors found in today’s solar panels to form “tandem” solar cells, which have the potential to outperform silicon solar cells in terms of theoretical efficiency.
“Silicon solar cells are great because they are very efficient and can last a long time, but the high efficiency comes at a high cost,” said Xiwen Gong, an assistant professor of chemical engineering at the University of Michigan. “To produce high-purity silicon, temperatures over 1,000 degrees Celsius are required. Otherwise, the efficiency will be lower.
Silicon solar cells are great because they are very efficient and can last a long time, but the high efficiency comes at a high cost. To produce high-purity silicon, temperatures over 1,000 degrees Celsius are required. Otherwise, the efficiency will be lower.
Xiwen Gong
The high temperature comes with higher economic and environmental costs. But while perovskites can be produced at lower temperatures, they degrade when exposed to heat, moisture and air. As a result, the lifespan of perovskite today is too short to be commercially competitive in solar panels.
Gong’s research aims to make hardier perovskite solar cells, and her latest study published in the journal Mattersuggests that bulky “defect pacifying” molecules are best at increasing the perovskites’ stability and overall lifespan.
Perovskite crystals contain lead atoms that aren’t fully bound to the other components within the perovskite. Such “undercoordinated sites” are defects often found on the crystal surfaces and at grain boundaries where there’s a break in the crystal lattice. These defects hinder the movement of electrons and speed up the decay of the perovskite material.
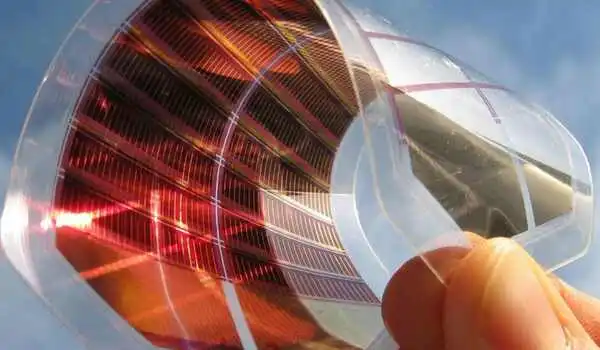
Engineers already know that mixing defect-pacifying molecules into the perovskites can help lock up the undercoordinated lead, in turn preventing other imperfections from forming at high temperatures. But until now, engineers didn’t know exactly how a given molecule affected the hardiness of perovskite cells.
“We wanted to figure out what features on the molecules specifically improve the perovskite’s stability,” said Hongki Kim, a former postdoctoral researcher in chemical engineering and one of the study’s first authors.
To investigate the problem, Gong’s team created three additives with a range of shapes and sizes and added them into thin films of perovskite crystals, which can absorb light and convert it to electricity. Each additive contained the same or similar chemical building blocks, which made size, weight and arrangement the main properties differentiating them.
Then, the team measured how strongly the different additives interacted with perovskites and consequently influenced the formation of defects in the films. Larger molecules by mass were better at sticking to the perovskite because they had more binding sites that interact with perovskite crystals. As a result, they tended to be better at preventing defects from forming.
But the best additives also needed to take up a lot of space. Large but skinny molecules resulted in smaller perovskite grains during the manufacturing process. Smaller grains aren’t ideal because they also create perovskite cells with more grain boundaries, or more areas for defects to form. In contrast, bulky molecules forced larger perovskite grains to form, which in turn reduced the density of grain boundaries in the film.
Heating the perovskite films to temperatures beyond 200 degrees Celsius indicated that bulky additions helped the films keep their unique slate black color and generate fewer structural flaws.
“Both the size and configuration are important when designing additives, and we believe this design philosophy could be implemented across various perovskite formulations to further improve the lifetime of perovskite solar cells, light emitting devices, and photodetectors,” said Carlos Alejandro Figueroa Morales, a doctoral student in macromolecular science and engineering and one of the study’s first authors.