BTB (bromothymol blue) is a pH indicator. It is a dye that is used as a pH indicator. Bromthymol blue is an acid with a low pH. Depending on the pH of the solution, it can be acidic or basic. In acidic solutions, this reagent is yellow, blue in basic solutions, and green in neutral solutions. It contains bromothymol blue, a pH indicator that causes the medium to change from yellow to blue-green; antimicrobial agents chloramphenicol and chlortetracycline; and an antimycotic agent cycloheximide.
It is mostly used in applications that call for measuring substances with a relatively neutral pH. (near 7). One common application is to detect the presence of carbonic acid in a liquid.
Properties
In a solution, bromothymol blue acts as a weak acid. It can thus be protonated or deprotonated, and thus appear yellow or blue. It has a bright aquamarine color on its own and a greenish-blue color in a neutral solution. The deprotonation of the neutral form produces a highly conjugated structure, which accounts for the color difference. The greenish color in neutral solution is caused by an intermediate of the deprotonation mechanism.
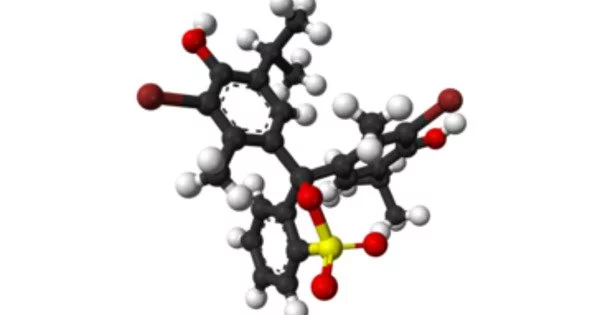
- Chemical formula: C27H28Br2O5S
- Molar mass: 624.38 g·mol−1
- Density: 1.25 g/cm3
- Melting point: 202 °C (396 °F; 475 K)
- Solubility in water: Sparingly soluble in water
- Acidity (pKa): 7.0
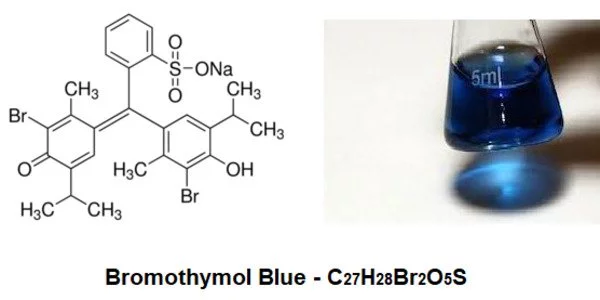
Structure
The protonated form of bromothymol blue has a peak absorption at 427 nm, transmitting yellow light in acidic solutions, and the deprotonated form has a peak absorption at 602 nm, transmitting blue light in more basic solutions. Highly acidic Bromothymol blue is a magenta color. The general carbon skeleton of bromothymol blue is shared by many indicators, including chlorophenol red, thymol blue, and bromocresol green.
Bromothymol blue’s active indication range of pH 6.0 to 7.6 is due to the presence of one moderate electron-withdrawing group (bromine atom) and two moderate donating groups (alkyl substituents). While conjugation determines the length and nature of the color change range, these substituent groups ultimately determine the active range of the indicator.
Synthesis and preparation
Bromothymol blue is synthesized by addition of elemental bromine to thymol blue in a solution in glacial acetic acid.
To prepare a solution for use as pH indicator, dissolve 0.10 g in 8.0 cm3 N/50 NaOH and dilute with water to 250 cm3. To prepare a solution for use as indicator in volumetric work, dissolve 0.1 g in 100 cm3 of 50% (v/v) ethanol.
Uses
Bromothymol blue can be used to monitor photosynthetic processes or as a respiratory indicator (turns yellow as CO2 is added). Exhaling through a tube into a neutral solution of BTB is a common demonstration of its pH indicator properties. As carbonic acid is formed by the absorption of carbon dioxide from the breath into the solution, the solution’s color changes from green to yellow. As a result, BTB is frequently used in science classes to demonstrate that the more muscles that are used, the more CO2 is produced.
Bromothymol blue and phenol red have been used to monitor fungal asparaginase enzyme activity, with phenol red turning pink and bromothymol blue turning blue indicating an increase in pH and thus enzyme activity. According to a recent study, methyl red is more useful in determining activity because of the bright yellow ring form in the zone of enzyme activity.
It can also be used as a biological slide stain in the laboratory. It is already blue at this point, and a few drops are used on a water slide. With the blue coloring mixed in, the cover slip is placed on top of the water droplet and specimen. It is sometimes used to define cell walls or nuclei under the microscope.