Scientists have discovered the genetic regulation of a model plant’s immune response, which is essential for agriculture and food safety. In monocots (plants with a single embryonic leaf), the mechanism of plant defense mediated by the non-expressor of pathogenesis-related (NPR) genes remains unknown.
In the model monocot Brachypodium distachyon, scientists from Tokyo University of Science have uncovered how the NPR family of genes govern immunological responses. These discoveries provide a roadmap for plants’ defensive systems and could lead to greater study into robust crop species, perhaps enhancing pesticide-free cereal crop production.
Plants are split into two groups: dicotyledonous and monocotyledonous. Apart from differences in embryonic anatomy, these groupings have a number of other characteristics in common. As a result, it’s probable that their immune responses to different threats will differ as well.
Immune responses in plants? Leaves you confused?
Plants, like people, respond to external threats, despite their immune systems being organized and functioning very differently than ours. These immunological responses have been extensively examined in dicot models, whereas monocots have received less attention. During a pathogen attack, the non-expressor of pathogenesis-related (NPR) family of genes is known to influence defense signals.
NPR1 (AtNPR1) is a binding site for salicylic acid (SA) in Arabidopsis thaliana, a dicot, and interacts with the TGA group of transcription factors (TFs), which are responsible for turning genes on and off as needed. This turns on defense genes including pathogenesis-related protein 1 (PR-1) that regulate the plant’s immune response.
Does this happen in monocots as well?
Prof. Gen-ichiro Arimura of Tokyo University of Science in Japan headed a research team to find out. They recognized that several monocots, such as rice and wheat, had a similar NPR1-mediated immune response when they are attacked by a pathogen.
Certain amino acid residues-especially those of arginine (Arg)-are responsible for SA binding in Arabidopsis NPRs. So, our mutant NPR2 was missing a specific arginine residue-Arg468.
Prof. Arimura
However, the researchers suspected that other monocots would react differently, therefore they wanted to look at additional NPRs like AtNPR3/AtNPR4, which could have the opposite impact as NPR1.
As a result, Prof. Arimura and his colleagues chose the model monocot Brachypodium distachyon, also known as the southern duckweed, to study NPR function and immune response.
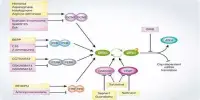
NPR genes in B. distachyon regulate TGA-promoted transcription of defense-responsive genes, according to their research, which was published in The Plant Journal.
The researchers first found and cloned monocot B. distachyon’s NPR genes-BdNPR1, 2, and 3-which were similar to NPR sequences in other dicot species, including Arabidopsis. BdNPR2 expression increased considerably in response to methyl salicylate treatment, but not BdNPR1/BdNPR3, indicating that it plays a favorable function in plant defense response.
By examining gene expression and molecular interactions in B. distachyon protoplasts, the researchers were able to demonstrate that one of the BdNPRs (BdNPR2) activated BdTGA-1 in B. distachyon (exactly like other plants).
These investigations indicated that BdTGA1 and BdNPR2 interacted to upregulate PR-1 expression, confirming NPR2’s role in the immune response of B. distachyon.
Was this response mediated by SA? Another pertinent question, which the team answered by creating a created a mutant NPR2 gene. Prof. Arimura points out: “Certain amino acid residues-especially those of arginine (Arg)-are responsible for SA binding in Arabidopsis NPRs. So, our mutant NPR2 was missing a specific arginine residue-Arg468.”
This mutant was less successful at boosting PR-1 expression than the normal wild-type NPR2, showing that Arg468 was required for SA binding on NPR2, which elevated PR-1. BdNPR1 inhibited this increase in their experimental experiments, implying that it functions as an immunological inhibitor in B. distachyon.
Prof. Arimura sums it all up for us. “When the plant is in a healthy state, BdNPR1 may stop BdNPR2 from activating BdTGA1, keeping the PR1 gene turned off. But when the plant is attacked by a pathogen, SA levels rise and stimulate BdNRP2 expression, which then cascades, and ‘turns on’ the PR1 gene.”
Surprised by how functionally unique BdNRP2 is, Prof. Arimura explains that the “sequence similarities between NPR2 from B. distachyon and other plants does not affect their functions which are distinctively different for every plant species.”
But how does this genetic research translate into real-life applications? Monocots include several essential crops such as wheat and rice. Pesticides are used to protect these plants, which are susceptible to microbiological infections and pests. The pesticides wreak havoc on the environment.
“This vicious cycle can be broken by understanding monocots’ defense systems, and addressing their susceptibility in a more sustainable way, with pesticide-free cultivation,” says Prof. Arimura, who hopes this research will be used to further plant biotechnology. It moves us closer to solving global environmental and food security problems, allowing us to work toward a more sustainable society.