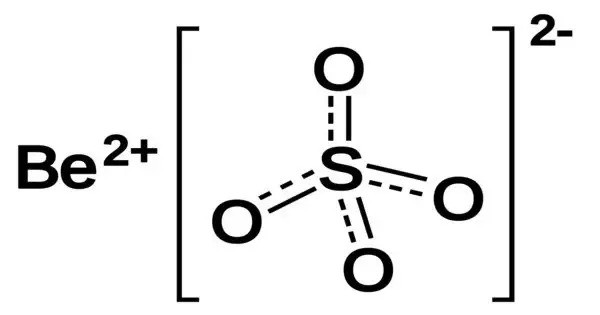
Beryllium sulfate normally encountered as the tetrahydrate, [Be(H2O)4]SO4 is a white crystalline solid. It is an inorganic compound, typically appearing as a white crystalline solid or colorless tetrahydrate. It was first isolated in 1815 by Jons Jakob Berzelius. It is used in niche applications, such as in the production of beryllium compounds and as a reagent in analytical chemistry. However, its use is limited due to beryllium’s extreme toxicity.
Beryllium sulfate may be prepared by treating an aqueous solution of many beryllium salts with sulfuric acid, followed by evaporation of the solution and crystallization. The hydrated product may be converted to anhydrous salt by heating at 400 °C. It is formed by the reaction of beryllium oxide or hydroxide with sulfuric acid. Highly soluble in water, it dissociates to release beryllium ions (Be²⁺) and sulfate ions (SO₄²⁻), with the tetrahydrate being the most common form due to beryllium’s tendency to form hydrated complexes.
Properties
- Chemical formula: BeSO4
- Molar mass: 105.075 g/mol (anhydrous), 177.136 g/mol (tetrahydrate)
- Appearance: white solid
- Odor: odorless
- Density: 2.44 g/cm3 (anhydrous), 1.71 g/cm3 (tetrahydrate)
- Melting point: 110 °C (230 °F; 383 K) (tetrahydrate, −2H2O)
- Boiling point: 2,500 °C (4,530 °F; 2,770 K) (anhydrate), 580 °C (tetrahydrate)
- Solubility in water: 36.2 g/100 mL (0 °C), 54.3 g/100 mL (60 °C)
- Solubility: insoluble in alcohol
- Refractive index (nD): 1.4374 (tetrahydrate)
Natural Occurrence
Rare in nature – Beryllium sulfate does not commonly occur as a mineral.
Beryllium is primarily found in beryl (Be₃Al₂Si₆O₁₈) and bertrandite (Be₄Si₂O₇(OH)₂), from which it is extracted and then reacted with sulfuric acid to form BeSO₄.
Industrial Production:
Produced typically by reacting beryllium oxide (BeO) or beryllium carbonate with sulfuric acid:
BeO+H2SO4 → BeSO4 + H2O
Uses
- Research & chemical analysis: Used in some chemical analysis procedures.
- Material science: Can serve as a precursor for beryllium-containing materials.
- Limited due to toxicity: Beryllium compounds are toxic and strictly regulated.
Health and Safety
- Toxicity: Beryllium compounds are highly toxic and can cause chronic beryllium disease (CBD) upon inhalation.
- Handling: Requires protective equipment and proper ventilation.
- Environmental hazard: Harmful to aquatic life in large concentrations.