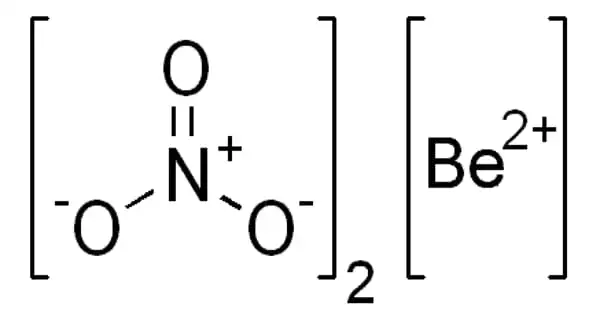Beryllium nitride (Be3N2) is a beryllium nitride. It can be synthesized from the elements at high temperatures (1100–1500°C), but unlike Beryllium azide or BeN6, it decomposes in a vacuum into beryllium and nitrogen. It is easily hydrolyzed, yielding beryllium hydroxide and ammonia. It exists in two polymorphic forms: cubic α-Be3N2 with a defect anti-fluorite structure and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4, in an ammonia stream to form BeSiN2 at 1800–1900°C.
Properties
- Compound Formula: Be3N2
- Molecular Weight: 55.0499
- Appearance: yellow or white powder
- Melting Point: 2200 °C
- Boiling Point: 2240 °C (decomposes)
- Density: 2.71 g/cm3
- Solubility in H2O: N/A
Preparation
Beryllium nitride is made by heating beryllium metal powder with dry nitrogen in an oxygen-free environment at temperatures ranging from 700 to 1400°C. It is a beryllium and nitrogen compound that is made by heating beryllium metal powder with dry nitrogen at high temperatures (1100-1500 °C). It is a relatively reactive substance that oxidizes quickly in air at 600°C and decomposes in water.

Reactions
Beryllium nitride has the molecular formula of Be3N2 and a molecular weight of 55.0652 g/mol. It can be prepared from the elements at high temperatures (1100– 1500 °C). Be3N2 decomposes in a vacuum into beryllium and nitrogen:
3Be +N2 → Be3N2
It is a white to yellow powder with a density of 2.71 g/cm3. Its melting point is 2208°C and a boiling point of 2240°C. Its CAS number is 1304-54-7. It is soluble in water and is readily hydrolyzed forming beryllium hydroxide and ammonia:
Be3N2 + 3H2O3 → Be(OH)2 + 2NH3
Beryllium nitride reacts with mineral acids producing ammonia and the corresponding salts of the acids:
Be3N2 + 6 HCl → 3 BeCl2 + 2 NH3
In strong alkali solutions, a beryllate forms, with the evolution of ammonia:
Be3N2 + 6 NaOH → 3 Na2BeO2 + 2 NH3
Both the acid and alkali reactions are brisk and vigorous. Reaction with water, however, is very slow:
Be3N2 + 6 H2O → 3 Be(OH)2 + 2 NH3
Reactions with oxidizing agents are likely to be violent. It is oxidized when heated at 600 °C in the air.
Uses
It is used in refractory ceramics as well as in nuclear reactors and to produce radioactive carbon-14 for tracer applications.