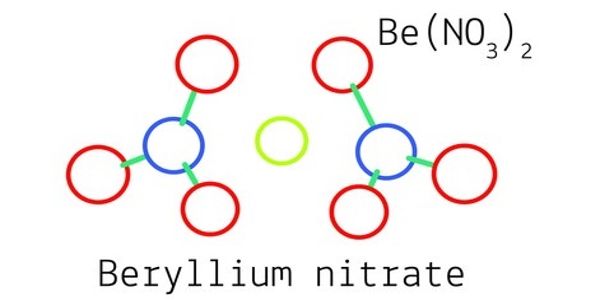
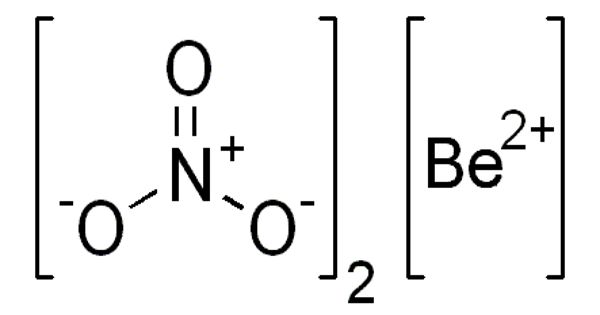Beryllium nitrate is a solid, easily soluble in water, with a weak oxidizing effect. It is also known as beryllium dinitrate, which is an ionic beryllium salt of nitric acid with the chemical formula Be(NO3)2. Each formula unit is composed of one Be2+ cation and two NO3– anions. The commercial product is offered as a solution in water or nitric acid.
Properties
Beryllium nitrate is a white to pale yellow crystalline solid. It is soluble in water. It is noncombustible, but it will accelerate the burning of combustible materials. Beryllium chloride, BeCl2, melts at 405°C and boils at 520°C.
- Molecular Weight: 133.02
- Appearance: White
- Melting Point: 60° C (140° F)
- Boiling Point: 100° C (212° F)
- Density: 1.56 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 132.988
- Monoisotopic Mass: 132.988
Hazards
Beryllium is considered extremely toxic by most chemists, placing it right next to plutonium. Beryllium nitrate is a toxic chemical, like all other beryllium compounds. If large quantities of the material are involved or the material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion. It is also an irritant in small doses. When burned, it gives off irritating or toxic fumes. Toxic oxides of nitrogen are produced in fires involving this material. It is used in chemical analysis. However, when massive short-term exposure occurs, acute pneumonitis can set in, but symptoms do not manifest themselves for 3 days. In fact, exposure to beryllium could cause a chronic form of a lung disease called beryllium disease (CBD), with symptoms similar to sarcoidosis.
Preparation
Beryllium nitrate can be prepared by reacting beryllium hydroxide in nitric acid.
Be(OH)2 + 2 HNO3 → Be(NO3)2 + 2 H2O
Use
Beryllium is used as a reflector material, in research and fast reactors, as well as in power reactors, for moderators, reflectors and fuel cladding material. Beryllium nitrate is used in gas and acetylene lamps. It is a compound that forms colorless, deliquescent crystals that are soluble in water; used to introduce beryllium oxide into materials used in incandescent mantles.
Information Source: