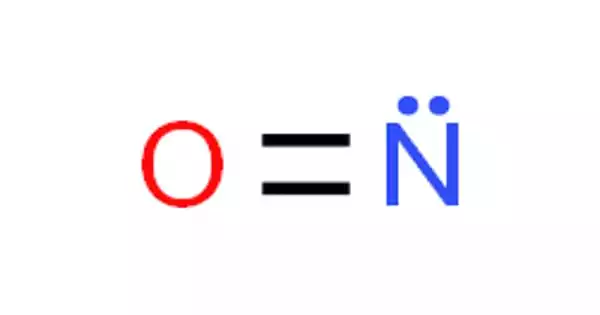
Beryllium monohydride (BeH) is an example of a molecule with a half-bond order according to molecular orbital theory. It is an inorganic compound consisting of one beryllium atom and one hydrogen atom. It is a metastable monoradical species which has only been observed in the gas phase. In beryllium monohydride, beryllium has a valency of one, and hydrogen has a valency of one.
It is a highly reactive and unstable molecule that exists mainly in the gas phase or under specific experimental conditions. The compound is notable for its simplicity and serves as an important subject in quantum chemistry and molecular structure studies due to its lightness and unique bonding nature.
In laboratory conditions, Beryllium Monohydride can be generated by high-temperature reactions of beryllium metal with hydrogen gas or by laser ablation techniques. However, it decomposes readily upon cooling or in the presence of air and moisture, forming beryllium oxide (BeO) or beryllium hydroxide (Be(OH)₂).
Properties
BeH has a molecular weight of about 10.02 g/mol and is typically observed as a diatomic molecule. It exhibits covalent bonding, though the bond is relatively weak compared to other metal hydrides because beryllium lacks sufficient d-orbitals for effective electron sharing. The molecule has a linear geometry and a bond length of approximately 1.34 Å.
- Chemical formula: BeH•
- Molar mass: 10.02012 g mol−1
- Appearance: Colourless gas
- Bond type: Covalent, with some ionic character due to the electronegativity difference between Be and H.
- Molecular structure: Linear molecule with a short Be–H bond (~1.34 Å).
- Stability: Highly reactive and thermally unstable; decomposes easily into elemental beryllium and hydrogen.
- Magnetic properties: It has a doublet ground state due to one unpaired electron (Be in +1 oxidation state).
- Spectral properties: Observed through microwave and laser spectroscopy, useful for quantum chemistry studies.
BeH has only 5 electrons and is the simplest open shell neutral molecule, and is therefore extremely important for the benchmarking of ab initio methods. With such a light mass, it is also an important benchmark system for studying the breakdown of the Born–Oppenheimer approximation. Due to its simplicity, BeH is expected to be present in astronomical contexts such as exoplanetary atmospheres, cool stars, and the interstellar medium, but so far has only been found on the Sun.
Occurrences
Beryllium Monohydride does not occur naturally on Earth due to its instability. It is generally produced in laboratory conditions via high-temperature reactions between beryllium vapor and hydrogen gas or through laser ablation techniques. In astrophysical contexts, BeH molecules have been detected in stellar atmospheres, such as in cool stars, providing insights into stellar chemistry and molecular formation in extreme environments.