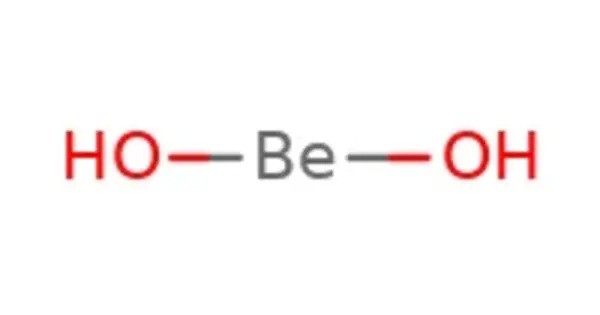
Beryllium hydroxide [Be(OH)₂] is an inorganic compound and the main hydroxide of beryllium. It is an amphoteric hydroxide, dissolving in both acids and alkalis. It typically appears as a white, amorphous or crystalline solid, insoluble in water but soluble in acids and alkalis, showing its amphoteric nature. It can react with acids to form beryllium salts, such as beryllium sulfate, and with strong bases to form beryllates, like sodium beryllate.
Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic). When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates. This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers.
Properties
It typically appears as a white, amorphous, gelatinous solid. It is amphoteric, meaning it can react with both acids and bases, forming beryllium salts with acids and beryllates with strong bases. Its solubility in water is very low, but it dissolves in both acidic and alkaline solutions. It is stable at room temperature but decomposes upon heating to beryllium oxide (BeO) and water.
- Chemical formula: BeH2O2
- Molar mass: 43.026 g·mol−1
- Appearance: Vivid white, opaque crystals
- Density: 1.92 g cm−3
- Melting point: (decomposes)
- Solubility in water: 0.0000023965 g/L
Reactions
Beryllium hydroxide is difficult to dissolve in water. With alkalis it dissolves to form the tetrahydroxoberyllate (also known as tetrahydroxidoberyllate) anion, [Be(OH)4]2−. With sodium hydroxide solution:
2 NaOH(aq) + Be(OH)2(s) → Na2[Be(OH)4](aq)
With acids, beryllium salts are formed. For example, with sulfuric acid, H2SO4, beryllium sulfate is formed:
Be(OH)2 + H2SO4 → BeSO4 + 2 H2O
Beryllium hydroxide dehydrates at 400 °C to form the soluble white powder, beryllium oxide:
Be(OH)2 → BeO + H2O
Further heating at higher temperature produces acid insoluble BeO.
Production
Beryllium hydroxide is most commonly produced by adding alkali to soluble beryllium salts (such as beryllium sulfate or nitrate) or by hydrolysis of beryllium compounds. It exists in several hydrated and crystalline forms depending on preparation methods and conditions.
Beryllium hydroxide is toxic and must be handled with extreme caution. Inhalation or ingestion can cause berylliosis, a chronic and potentially fatal lung disease. Despite its hazards, it is critical in nuclear, aerospace, and electronics industries due to its role in beryllium processing.
Occurrences
Beryllium hydroxide does not occur naturally in free form but can be synthesized from beryllium-containing ores, such as beryl (Be₃Al₂(SiO₃)₆) and bertrandite (Be₄Si₂O₇(OH)₂). Industrially, it is produced by treating soluble beryllium salts (e.g., beryllium sulfate) with alkali, precipitating Be(OH)₂. It also serves as an intermediate in the production of beryllium oxide and metallic beryllium.