Beryllium carbide, abbreviated Be2C, is a metal carbide. It is extremely hard, similar to diamond. It is utilized as the core material in nuclear reactors. It is a chemical compound made of beryllium and carbon. It is a very hard ceramic substance with high melting and boiling temperatures.
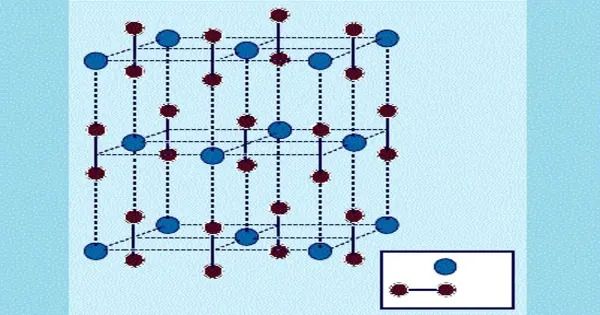
It is a refractory chemical, which means it can tolerate extreme temperatures without degrading. It forms a hexagonal crystal shape. Each beryllium atom is connected to two carbon atoms, resulting in a trigonal planar configuration around the beryllium atoms.
Properties
- Chemical formula: CBe2
- Molar mass: 30.035 g·mol−1
- Appearance: Yellow to red crystals
- Odor: odorless
- Density: 1.90 g cm−3 (at 15 °C)
- Melting point: 2,100 °C (3,810 °F; 2,370 K) (decomposes)
- Solubility in water: decomposes
- Crystal structure: cubic
Preparation
Beryllium carbide is prepared by heating the elements beryllium and carbon at elevated temperatures (above 950°C). It also may be prepared by reduction of beryllium oxide with carbon at a temperature above 1,500°C:
2BeO + 3C → Be2C + 2CO
Beryllium carbide decomposes very slowly in water and forms methane gas:
Be2C + 2H2O → 2BeO + CH4
The rate of decomposition is faster in mineral acids with evolution of methane.
Be2C + 4 H+ → 2 Be2+ + CH4
In hot concentrated alkali the reaction is very rapid, forming alkali metal beryllates and methane:
Be2C + 4OH− → 2 BeO22− + CH4
Uses
Beryllium carbide has potential applications in materials science and engineering, particularly in the development of advanced ceramics and as a semiconductor material. However, its use is limited due to the toxicity of beryllium compounds.
Occurrence
Beryllium carbide is not found naturally but can be synthesized by reacting beryllium oxide (BeO) with carbon at high temperatures.
Toxicity
Beryllium compounds, including beryllium carbide, are toxic and can pose health risks if inhaled or ingested. Proper handling procedures and safety precautions are necessary when working with beryllium-containing materials.