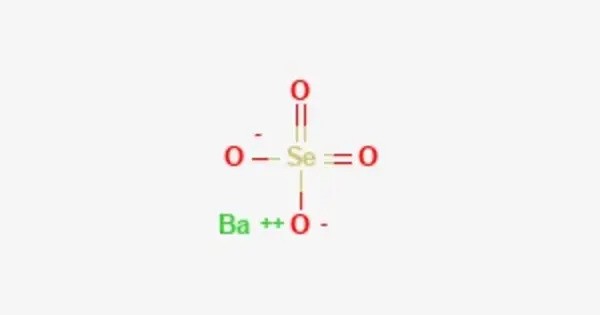
Barium selenate is an inorganic compound with the chemical formula BaSeO4. It is a barium salt of selenic acid. In its pure form, it’s a white, crystalline powder. It is isomorphous with barium sulfate, but its solubility is 18 times that of barium sulfate, and its thermal stability is worse than that of barium sulfate. It can be used as a reagent in analytical chemistry for detecting certain substances.
It is used as a reagent in qualitative analysis to test for the presence of selenate ions. In some industrial processes, barium selenate is used as a pigment or in the production of certain types of glass. It has applications in electronics, particularly in the manufacturing of certain types of electronic components.
Preparation
Barium selenate can be obtained from the reaction of any soluble barium salt and sodium selenate:
BaCl2 + Na2SeO4 → BaSeO4↓ + 2 NaCl
Properties
Barium selenate is a white solid that is slightly soluble in water. When heated above 425 °C, the compound decomposes. Another barium selenate, barium diselenate, BaSe2O7, is also known. It has an orthorhombic baryte-type crystal structure with the space group Pnma (space group no. 62) (a = 8.993 Å, b = 5.675 Å, c = 7.349 Å).
Properties
- Chemical formula: BaSeO4
- Molar mass: 280.29
- Appearance: colourless crystals
- Solubility in water: 0.0118 g (20 °C), 0.0138 g (100 °C)
- Molecular Weight: Approximately 253.24 g/mol
- Density: Around 4.3 g/cm³
- Melting Point: Decomposes at high temperatures rather than having a distinct melting point
Uses
Barium selenate has been used as a “slow release” source of selenium for grazing animal feed crops and was intended to ensure selenium supply to grazing animals. In Switzerland and the EU, direct use as a feed additive is prohibited. Barium selenide can be obtained by reducing barium selenate in a hydrogen stream:
BaSeO4 + 4 H2 → BaSe + 4 H2O
Safety Considerations
Barium selenate should be handled with care as both barium and selenium compounds can be toxic. Proper safety measures, including personal protective equipment and working in a well-ventilated area, are essential to avoid exposure.