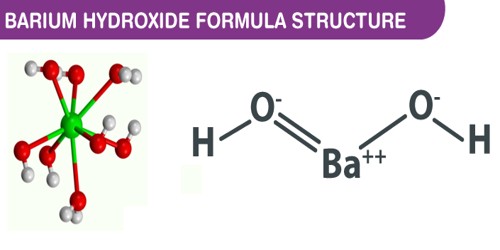
Barium hydroxide is a chemical compound. Its chemical formula is Ba(OH)2. It has a molecular weight of 121.6324 g/mol. It contains barium and hydroxide ions. It occurs as an anhydrate whose prismatic, colorless crystals are very deliquescent. It is commonly found in nature, as present in many minerals.
Properties
Barium hydroxide is a white, odorless solid. It dissolves in water to make a basic solution. It decomposes to barium oxide when heated in a vacuum. It reacts with carbon dioxide to make barium carbonate. It reacts with any sulfate to make barium sulfate. It reacts with hydrogen sulfide to make barium sulfide. The three forms are slightly soluble in water at low temperature but the solubility in water increases with the temperature.
- Compound Formula: Ba(OH)2.
- Molecular Weight: 171.36
- Appearance: White to off-white powder, crystals or crystalline powder
- Melting Point 78° C (172.4° F)
- Boiling Point 780° C (1,436° F)
- Density 3.74 g/cm3
- Solubility in H2O N/A
Preparation
It is made by dissolving barium oxide in water. It can be prepared by dissolving barium oxide (BaO) in water:
Uses
Barium hydroxide is used in titrations. If sodium hydroxide solution is used, some sodium carbonate may be in there. It is less basic than sodium hydroxide, so the titration can be messed up. When barium hydroxide reacts with carbon dioxide to make barium carbonate, it forms a white solid that can be removed.
It is used as a source of barium to produce other barium salts as barium phosphate, barium sulfide and barium sulfate that are using in the chemical industry in diverse processes.
It is used as a strong base in organic chemistry. It is used to clean up sulfuric acid spills by making barium sulfate. It can also be used for removing ink marks from clothes.
Safety
Barium hydroxide is corrosive because it is basic. It is harmful if swallowed or inhaled. It is also toxic because of barium ions. It can be corrosive to metals.
Information Source: