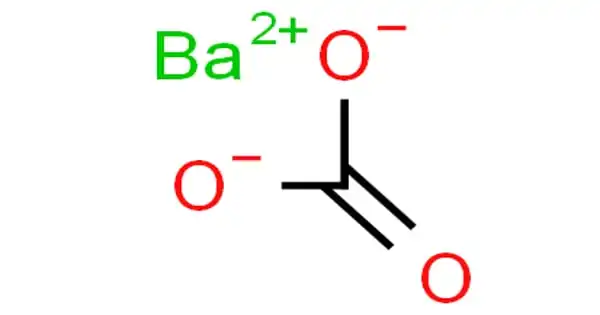
Barium carbonate is a white solid that forms when barium hydroxide and urea react. It is an inorganic compound with the formula BaCO3. It is a white salt that is poorly soluble in water, like most alkaline earth metal carbonates. It is found as the mineral witherite. It is one of the most important barium compounds in terms of commerce.
Barium Carbonate is generally toxic in nature and occurs in other forms such as a mineral form known as witherite, and it can also be precipitated from barytes. It can also be seen in turquoise glazes.
Properties
Barium carbonate is a white powder. It is insoluble in water and soluble in most acids, with the exception of sulfuric acid. It has a specific gravity of 4.275. is an ionic compound with +2 charge on the cation and -2 charge on the anion.
- Density: 4.29 g/cm³
- Molecular Weight/ Molar Mass: 197.34 g/mol
- Boiling Point: 1,360 °C
- Melting Point: 811 °C
- Odour: No odour
- Appearance: White crystals

Preparation
Barium carbonate is made commercially from barium sulfide with by treatment with sodium carbonate at 60 to 70 °C (soda ash method) or, more commonly carbon dioxide at 40 to 90 °C:
In the soda ash process, an aqueous solution of barium sulfide is treated with sodium carbonate:
BaS + H2O + CO2 → BaCO3 + H2S
The mineral witherite is also refined to produce barium carbonate. This is accomplished by first reacting witherite with an ammonium salt, resulting in a soluble barium salt, and then reacting the formed ammonium carbonate with the barium salt again to produce refined BaCO3.
Reactions
Barium carbonate reacts with acids such as hydrochloric acid to form soluble barium salts, such as barium chloride:
BaCO3 + 2 HCl → BaCl2 + CO2 + H2O
Pyrolysis of barium carbonate gives barium oxide.
Calcium salts that are soluble can react with Barium Carbonate to form Barium Sulphate which remains in solution and calcium carbonate. The related chemical reaction is given below:
BaCO3 + CaSO4 → CaCO3 + BaSO4
Barium Carbonate can react with Hydrochloric Acid to form Barium Chloride, Water, and Carbon Dioxide.
BaCO3 + 2HCl → BaCl2 + H2O + CO2
Uses
Although its whitish flour-like appearance has resulted in many barium poisoning cases, barium carbonate is widely used as a rodenticide. It is primarily used to remove sulfate impurities from chlor-alkali feedstock. Aside from that, it is a common precursor to barium-containing compounds such as ferrites.
Glass, oil-drilling, photographic, ceramic, enamel, barium magnetic materials, paint, brick, and chemical industries are among the major commercial applications of barium carbonate / BaCO3. It is a critical raw material in the manufacture of magnetic components and fiber optical glass.
Toxicity
It can be found in the mineral witherite and can also be precipitated from barytes. It is poisonous in nature. It’s also used in turquoise glazes. It should be kept at a low quality of less than 20% and protective measures should be taken when handling the chemical compound.