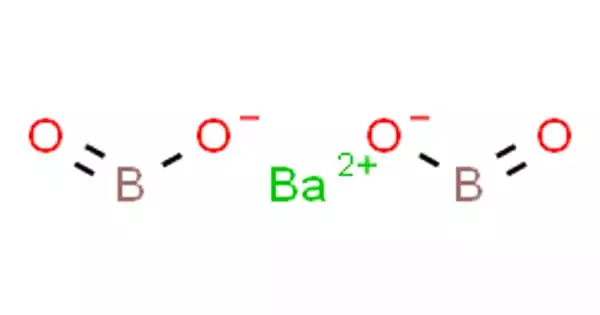
Barium borate is an inorganic compound, a barium borate with the chemical formula BaB2O4 or Ba(BO2)2. It is accessible in a hydrate or dehydrated form, as a white powder or colorless crystals. The crystals occur in two phases: high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.
Chen Chuangtian and colleagues from the Chinese Academy of Sciences’ Fujian Institute of Research on the Structure of Matter discovered and created barium borate.
Properties
- Molecular Weight: 222.95 g/mol
- Appearance: White powder or crystals
- Melting Point: 1095 °C
- Boiling Point: N/A
- Density: 3.85 g/cm3
- Morphology: Rhombohedral
- Solubility in H2O: N/A
In general, barium borate has a low acceptance angle, and hence, despite its higher optical nonlinearity, it is not considerably more efficient than other commonly accessible materials, particularly in the UV below 250 nm. On the other hand, it has a high damage threshold, is physically robust, has strong UV and IR transparency, and has outstanding average power capabilities. As an optical parametric amplifier, it enables deep UV creation and has enormous promise for generating tunable visible and IR light.
Applications
BBO is a well-known nonlinear optical crystal. Beta barium borate can generate quantum-linked photons. Barium borate is a bactericide as well as a fungicide. It’s used in paints, coatings, adhesives, plastics, and paper.
UV radiation does not affect barium borate. It has the ability to act as a UV stabilizer for polyvinyl chloride. When employed as a pigment, barium borate’s solubility is a drawback. There are silica-coated powders available. The alkaline and anodic passivation capabilities of the borate ion improve anti-corrosion performance.
Barium metaborate pigment is available in three grades: grade I is pure barium metaborate, grade II is a mixture of 27 percent zinc oxide and 18 percent calcium sulfate, and grade III is a mixture of 18 percent zinc oxide and 29 percent calcium sulfate. With zinc borate, barium borate performs synergistically.
In the level of about 2%, barium borate is utilized as a flux in various barium titanate and lead zirconate EIA Class 2 dielectric ceramic formulations for ceramic capacitors. The barium-boron ratio is crucial for flux performance; BaB2O2 concentration degrades flux performance.