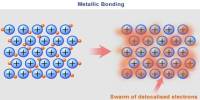
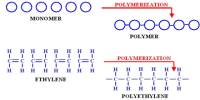
Basic purpose of this lecture is to describe on Atoms and Elements. Here focus on the Atomic Number and Mass Number Isotopes. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons. Finally describe on atomic Theory: Atoms are building blocks of elements Similar atoms in each element and Two or more different atoms bond in simple ratios to form compounds.
More Posts
-

Coasts and Reefs: Shallow Marine Processes
-

Business Continuity Planning (BCP)
-

Lost Continent of Balkanatolia May Have Been a Battleground for Ancient Mammals
-

Marketable Mobile Application
-
A Newly Discovered Protein Controls the Formation of Cellulose in Plant Cells
-

Annual Report 2017 of Shasha Denims Limited
Latest Post
-

Chronic Back Pain could be avoided with just one everyday Behavior
-

Diiodosyl Sulfate – an inorganic compound
-

Scientists use Fungi to create Living Material
-

Cobalt Phosphate
-

Unexpected Gut research identifies a hidden cause of Liver Illness and Diabetes
-

Researchers Discovered a Novel Method for Converting Sunlight into Fuel