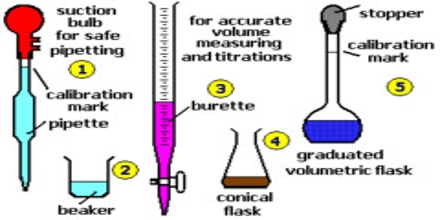
Basic objective of this lecture is to present on Titration or Volumetric Analysis. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of a known reactant. Because volume measurements play a key role in titration, it is also known as volumetric analysis. Titration is the slow addition of one solution of a known concentration to a known volume of another solution of unknown concentration until the reaction reaches neutralization, which is often indicated by a color change.
Titration or Volumetric Analysis