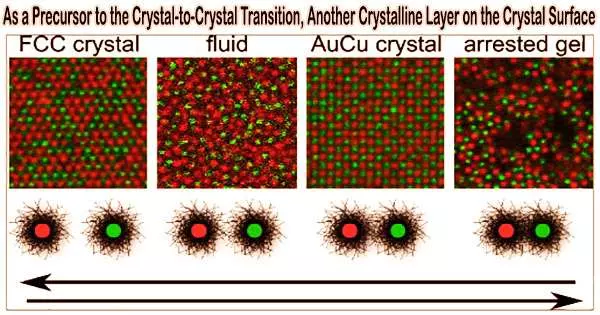
Below the freezing point of ice, or 0 degrees Celsius, there is a thin layer of water on its surfaces. Such a premelting process is crucial for snowflake development and skating. Similar to this, liquid frequently forms a thin layer of crystal on a flat surface before to freezing, or prefreezing.
As phase transition (such as melting and freezing) temperatures are approached, the thickness of the surface layer typically grows and diverges. It has rarely been investigated if analogous surface phenomena, such as premelting and prefreezing, exist as a precursor to a phase transition.
The pre-solid-solid transition is the hypothesis put out by Han’s team at HKUST that a polymorphic crystalline layer may emerge on a crystal surface prior to the crystal-crystal phase transition. It would be a so-called pre-solid-solid transition if carbon atoms close to a diamond’s surface could organise themselves into a graphite lattice before reaching the diamond-graphite transition temperature.
Premelting and prefreezing have a similar mechanism in that the freshly generated surface layer reduces the crystal’s surface energy. Han’s team made the observation that it is feasible when two polymorphic crystals to create a coherent interface, meaning that the two lattices have the correct lattice spacing and orientations at the interface.
As a result, because the newly formed crystal-crystal interface is coherent and requires essentially no energy, the surface of the denser polymorphic crystal can generate a layer of less dense crystal.
The pre-solid-solid transition was further confirmed by Han’s team through experiment and computer simulation. They discovered that because their interface is coherent, the surface of a thin-film colloidal crystal with a triangular lattice can create a square lattice. Similar to premelting, the thickness of the surface layer increases with temperature under a power law. Their simulation about atoms with different interactions further confirms these.
Our work shows that colloids are also effective in discovering new type of phenomenon even for the well-known phase behaviors under thermal equilibrium. Its mechanism is simple and thus could be proposed decades ago, but seems to be a blind spot in material science. One future direction is to search for this phenomenon in atomic or molecular crystals. Unlike premelting generally exists in most crystals, pre-solid-solid transition can only exist in polymorphic crystals with low-energy coherent interface. We suggested several candidates of atomic and molecular crystals with coherent interfaces.
Han
Premelting has been firstly conjectured by Michael Faraday, the father of electricity, in 1842, but not experimentally confirmed unambiguously until 1980s. The second type of phenomenon, prefreezing, has been proposed and observed in 1950s-70s. The pre-solid-solid transition that Han’s team hypothesized and discovered is the third kind of surface wetting phase transition precursor.
They discover that, while being a phenomenon in thermal equilibrium, surface crystalline layer can also exist in nonequilibrium processes following a sharp temperature shift, such as melting, freezing, and polycrystal annealing.
The surface layer makes the operations easier, which makes it useful for processing and fabricating materials. The polycrystal annealing, phase rule, or other evidence rule out the trivial solid-solid transition that happens on the free surface after crossing the solid-solid transition point.
Additionally, under the overlapped temperature regime of premelting and pre-solid-solid transition, they discovered the unprecedented double surface layers of liquid and square lattice.
Colloids, like milk, paint, and blood, are typically liquid suspensions of tiny, Brownian-moving particles having a diameter of less than one micron. Even inside the 3D bulk of crystals or liquids, optical microscopy can trace the particle thermal-motion paths, which is difficult to achieve in atomic systems. Colloids have thus been employed as a strong model system to assess the microscopic mechanisms behind phase transitions.
“Our work shows that colloids are also effective in discovering new type of phenomenon even for the well-known phase behaviors under thermal equilibrium.” Han said, “Its mechanism is simple and thus could be proposed decades ago, but seems to be a blind spot in material science. One future direction is to search for this phenomenon in atomic or molecular crystals. Unlike premelting generally exists in most crystals, pre-solid-solid transition can only exist in polymorphic crystals with low-energy coherent interface. We suggested several candidates of atomic and molecular crystals with coherent interfaces.”
The characteristics of polymorphic crystals vary frequently; for instance, diamond is hard, transparent, and non-conductive while graphite is soft, dark, and electrically conductive.
A crystal with a polymorphic crystalline layer would have adjustable surface characteristics, and such a layer might regrow after being worn or corroded off. Thus, it should be a very useful material in applications.
The work is funded by the Hong Kong Research Grants Council and the Guangdong Basic and Applied Research Foundation, and has been published recently in Nature Physics.