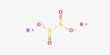
Arsenic trisulfide is the inorganic compound with the formula As2S3. It is a dark yellow solid that is insoluble in water. It occurs naturally as the mineral orpiment, which is bright yellow and has been historically used as a pigment and in traditional medicine. It also occurs as the mineral orpiment (Latin: auripigmentum), which has been used as a pigment called King’s yellow. It is produced in the analysis of arsenic compounds. It is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties.
Properties
- Chemical formula: As2S3
- Molar mass: 246.02 g·mol−1
- Appearance: yellow or orange crystals
- Density: 3.43 g/cm3
- Melting point: 310 °C (590 °F; 583 K)
- Boiling point: 707 °C (1,305 °F; 980 K)
- Solubility in water: insoluble
- Solubility: soluble in ammonia
Uses
- Pigments: Historically used as a yellow pigment called “king’s yellow” in paintings.
- Traditional Medicine: Used in some traditional medicine systems, though its toxicity limits its modern medical use.
- Semiconductors: Used in infrared optics and photonics due to its unique optical properties.
Toxicity
Arsenic compounds are generally toxic, and arsenic trisulfide is no exception. It is poisonous if ingested, inhaled, or if it comes into prolonged contact with skin. It can cause acute arsenic poisoning with symptoms like vomiting, abdominal pain, and diarrhea, and chronic exposure can lead to more severe health issues including cancer.
Handling and Safety
Proper safety measures should be taken when handling arsenic trisulfide, including wearing gloves, protective clothing, and using fume hoods to avoid inhalation of dust. Disposal must be done according to hazardous waste regulations to prevent environmental contamination.