Antimony is a silvery-white metal that is found in the earth’s crust. It is a chemical element with the symbol ‘Sb’ and atomic number 51. Antimony can be found in soils, waters, and air in very small amounts. Antimony will mainly pollute soils. Through groundwater, it can travel great distances towards other locations and surface waters. It is a moderately active element. It does not combine with oxygen in the air at room temperature. It is one of a number of the chemicals used today for high-quality industrial purposes that have a rich and elongated history.
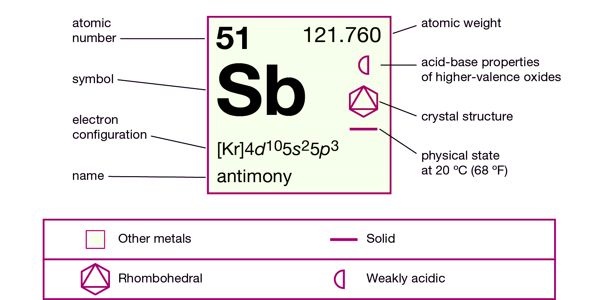
Antimony is a chemical element with the symbol Sb and atomic number 51. Classified as a metalloid, antimony is a solid at room temperature.
Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of the metal in the West was written in 1540 by Vannoccio Biringuccio.
Properties
It is a lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). It is a poor conductor of heat and electricity, it is stable in dry air and is not attacked by dilute acids or alkalis.
- Atomic number: 51
- Atomic mass: 121.75 g.mol-1
- Density: 6.684 g.cm-3
- Melting point: 631°C
- Boiling point: 1587°C
- Vanderwaals radius: 0.159 nm
Occurrences
Antimony is not an abundant element but is found in small quantities in over 100 mineral species. It is most often found as antimony(III) sulfide. For some time, China has been the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. China produces 88% of the world’s antimony. Other producers are Bolivia, Russia, and Tajikistan. The industrial methods for refining antimony are roasting and reduction with carbon or direct reduction of stibnite with iron. The United States produces antimony as a by-product at only one silver mine in Idaho.
Antimony compounds are prominent additives for chlorine and bromine-containing fire retardants found in many commercial and domestic products. An emerging application is the use of antimony in microelectronics.
Applications
Very pure antimony is used to make certain types of semiconductor devices, such as diodes and infrared detectors. Alloys of lead and tin with antimony have improved properties for solders, bullets, and plain bearings. Antimony is alloyed with lead to increase lead’s durability.
The largest applications for metallic antimony are an alloy with lead and tin and the lead antimony plates in lead–acid batteries. Antimony alloys are also used in batteries, low friction metals, type metal, and cable sheathing, among other products. Antimony compounds are used to make flame-retardant materials, paints, enamels, glass, and pottery.
Information Source: