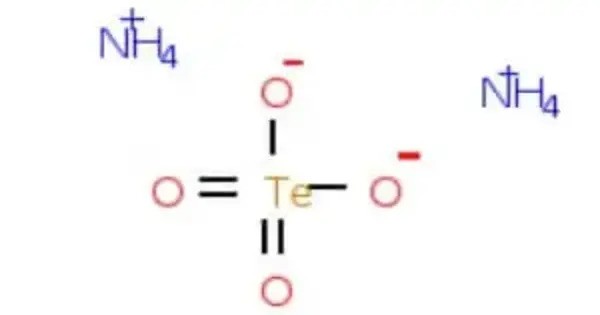
Ammonium tellurate is a chemical compound with the chemical formula (NH4)2TeO4. It consists of ammonium ions (NH₄⁺) and the tellurate anion (TeO₄²⁻), where tellurium is in the +6 oxidation state. It typically appears as a white to colorless crystalline solid and is soluble in water. The compound is prepared by reacting telluric acid (H₆TeO₆) with ammonium hydroxide.
Ammonium tellurate is mainly used in research settings, particularly in studies involving tellurium chemistry or as a precursor to other tellurium compounds. It is mildly toxic and should be handled with care, avoiding inhalation or ingestion. The compound is stable under standard conditions but may decompose upon strong heating.
Synthesis
The compound can be obtained by oxidation of tellurium dioxide TeO2 with hydrogen peroxide in an ammonia medium.
Properties
Ammonium tellurate forms white crystals, soluble in water.
- Chemical formula: H8N2O4Te
- Molar mass: 227.67 g·mol−1
- Appearance: White powder
- Density: 3.024 g/cm3
- Solubility in water: soluble
Chemical properties
Whrn heated, the compound decomposes releasing toxic fumes of Te, NOx, and NH3.
Natural Occurrence
Ammonium tellurate does not occur naturally. It is a synthetic compound made in laboratories or as a byproduct in certain tellurium processing operations.
Production
Commonly synthesized by:
H6TeO6 (telluric acid) + 2 NH3 → (NH4)2TeO4 + 2 H2O
Handling and Safety
- Hazard: Information
- Toxicity: Moderate – tellurium compounds can cause metallic taste, garlic odor on breath, and gastrointestinal distress
- Environmental Hazard: Potentially toxic to aquatic life
- Protective Measures: Use gloves, goggles, and work in a fume hood
Applications
- Analytical Chemistry: Rarely used in tellurium detection or separation methods.
- Research: Interest in tellurate compounds arises in materials chemistry and semiconductor research due to tellurium’s unique properties.
- Oxidation Chemistry: Occasionally explored for its oxidizing behavior in lab-scale experiments.