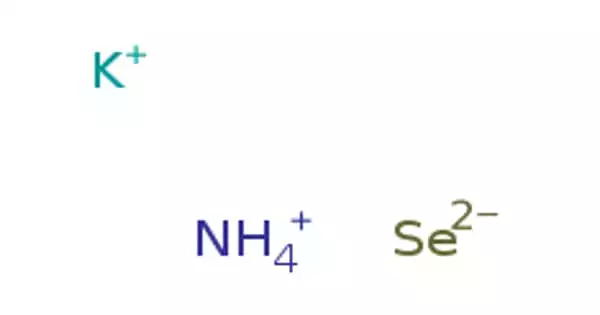
Ammonium selenide is a chemical compound with the formula (NH4)2Se. It is a white solid with selenium impurities that cause it to appear black. Other experiments have supported this. It has a salty taste. The compound dissolves easily in water but not in alcohol or acetone. It readily absorbs water, so if exposed to moist air, it will “scab” on damp surfaces.
It is a fertilizer that is sometimes used to make homemade explosives. During the purification process, it is crucial in the development of vaccines.
Properties
It exists as a translucent white crystalline solid and is salty in taste. One of the most vital usages is in fertilizers. It is a low-density inorganic compound, generally affordable. It has a molecular weight of 132.14gm/mol.
- Chemical formula: (NH4)2Se
- Molar mass: 115.05 g/mol
- Appearance: White solid
- Solubility in water: Reacts
- Solubility: Soluble in sodium hydroxide
Preparation
It was first claimed in 1898 that it could be made by reacting concentrated ammonia and hydrogen selenide gas. This was disproved in 1926, when it was discovered that ammonium selenide was unstable in water. Instead, anhydrous ammonia and hydrogen selenide gas (produced by the reaction of iron(II) selenide and hydrochloric acid) were used to create ammonium selenide. This compound, however, has no X-ray crystallography.
Reactions
Ammonium selenide reacts with water and various acids. For example, it reacts with nitric acid to form selenic acid:
(NH4)2Se + 4HNO3 + H2O → H2SeO4 + 3NH4NO3
It is a whitish solid crystallized compound with no odor. It is made up of the cations NH4(+) and SO4(-2). It is used as a food additive to “salt out” proteins through precipitation, in the examination of rubber grids, in the treatment of drinking water, as a flame retardant, acidity controller, pH controller, and so on. It is an inorganic salt that is soluble in water but not in alcohol, acetone, or ether.
Safety
It stimulates the eyes, skin, mucous membranes, and upper respiratory tract. Employees must be safeguarded. If the skin or eyes are touched, immediately rinse them with running water. To prevent moisture and rain, products are stored in a cool, ventilated warehouse. Keep a safe distance from any fire or heat source. It should be stored separately from acids and alkalis, and mixed storage and transportation should not be avoided.