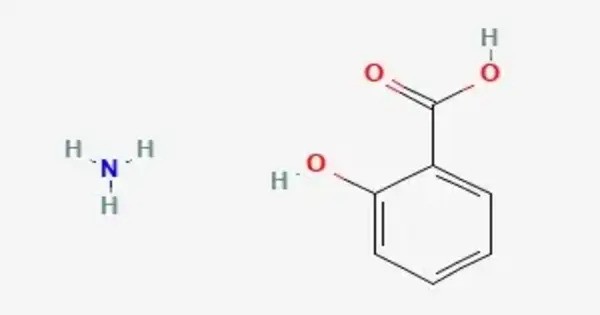
Ammonium salicylate is the ammonium salt of salicylic acid, widely used in pharmaceutical and dermatological formulations. It is a chemical compound with the chemical formula NH4C6H4(OH)COO. It is commonly used in topical creams, ointments, and lotions to relieve pain, itching, and discomfort associated with skin conditions such as rheumatism, arthritis, neuralgia, and muscular aches. As a counterirritant, it works by mildly irritating the skin, thereby increasing blood flow and reducing deeper pain sensations.
Ammonium salicylate also finds use in cosmetic and medicated skin care, especially in preparations aimed at softening hardened or thickened skin. Like other salicylates, it should be used with caution in individuals sensitive to aspirin or salicylic acid derivatives. Overuse may lead to local irritation or, rarely, systemic salicylate absorption.
Properties
Ammonium salicylate forms colorless crystals that are highly soluble in water and ethanol. The compound decomposes at 213°C. This compound combines the keratolytic, analgesic, and anti-inflammatory properties of salicylic acid with the solubility advantages provided by the ammonium ion. It appears as a crystalline or powdery substance, typically white to slightly off-white, and is soluble in water and alcohol. Its chemical formula is C7H9NO3, with a molecular weight of 155.15 g/mol.
- Chemical formula: C7H9NO3
- Molar mass: 155.153 g·mol−1
- Appearance: colorless powder
- Solubility in water: soluble
- Odor: Slight, characteristic of salicylates.
- Stability: Stable under normal conditions, but may decompose when heated strongly, releasing toxic fumes such as nitrogen oxides
- pH: Forms slightly acidic to neutral solutions depending on concentration.
Synthesis
The effect of ammonia solution on salicylic acid in an inert atmosphere:
NH3 + C6H4(OH)COOH → NH4C6H4(OH)COO
Occurrence in nature
It does not occur naturally; it is a synthetic compound produced by neutralizing salicylic acid with ammonium hydroxide.
Uses
The compound is commonly used in several industries, such as pharmaceuticals, cosmetics, and agriculture. As a drug, it is used in cases of topical skin diseases, relief of various muscle pains and as a component of several medications. Under exposure to light it discolors with the release of ammonia. It easily discolors iron compounds, and it forms a slightly acidic solution in water.