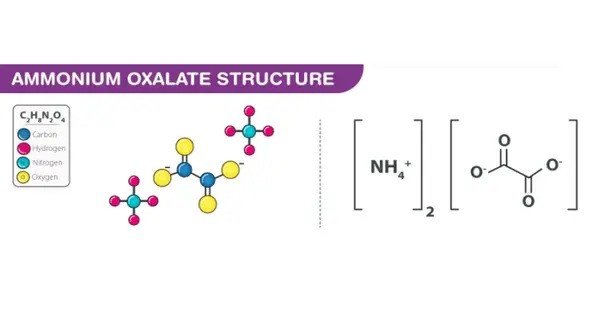
Ammonium oxalate is a chemical compound with the chemical formula [NH4]2C2O4. Its formula is often written as (NH4)2C2O4 or (COONH4)2. It is a white crystalline salt, is the ammonium salt of oxalic acid. It is an ammonium salt of oxalic acid. It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4).
The structure of ammonium oxalate is ([NH4]+)2[C2O4]2−. Ammonium oxalate sometimes comes as a monohydrate ([NH4]2C2O4·H2O). It is a colorless or white salt under standard conditions and is odorless and non-volatile. It occurs in many plants and vegetables.
Vertebrate
It is produced in the body of vertebrates by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine. It is a constituent of some types of kidney stone. It is also found in guano.
Properties
- Chemical formula: [NH4]2C2O4
- Molar mass: 124.096 g·mol−1
- Appearance: Colorless or white crystalline solid
- Density: 1.5 g/cm3
- Melting point: 70 C (158 F, 343.15 K)
- Solubility in water: 5.20 g/(100 ml) (25 °C)
- Decomposition: On heating, it decomposes to form ammonia, water, carbon monoxide, and carbon dioxide.
Chemical Behavior
- Decomposition: On heating, ammonium oxalate decomposes to form gases like ammonia (NH₃), nitrogen (N₂), and carbon dioxide (CO₂).
- Acid-base reactions: Acts as a weak acid due to the presence of oxalate.
- Complex formation: Oxalate ions can act as ligands to form coordination complexes with metals.
Natural Occurrence
- Found in some plants (as part of the oxalate group), though not usually as pure ammonium oxalate.
- Can be a component of kidney stones (calcium oxalate is more common).
Laboratory Uses
- Used as a reagent in analytical chemistry, especially for precipitating calcium ions.
- Used in the synthesis of other oxalates and coordination compounds.
Uses
- As a reagent in analytical chemistry (especially for detecting calcium ions, forming insoluble calcium oxalate).
- In biological research.
- Occasionally used in photography and textile dyeing.
- May appear naturally in some plants and biological samples.
Safety
- Can be toxic if ingested due to the oxalate ion, which can bind calcium in the body.
- Irritating to the skin, eyes, and respiratory tract.
- Should be handled with proper protective equipment.