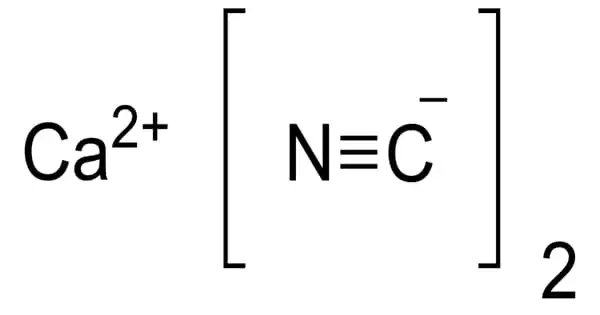Ammonium cyanide has the formula NH4CN and is an unstable inorganic compound. Ammonium cyanide is an inorganic chemical that is highly unstable. It is commonly used in organic synthesis. Because of its instability, it cannot be exported or sold commercially.
Cyanide is a chemical compound that has the CN group in its name. A carbon atom is triple-bonded to a nitrogen atom to form the cyano group.
Preparation
Ammonium cyanide is prepared in solution by bubbling hydrogen cyanide into aqueous ammonia at a low temperature –
HCN + NH3 (aq) → NH4CN (aq)
It may be prepared by the reaction of calcium cyanide and ammonium carbonate:
Ca(CN)2 + (NH4)2CO3 → 2 NH4CN + CaCO3
In dry state, ammonium cyanide is made by heating a mixture of potassium cyanide or potassium ferrocyanide with ammonium chloride and condensing the vapors into ammonium cyanide crystals:
KCN + NH4Cl → NH4CN + KCl
Properties
- Melting point: 36°C
- Boiling point: 31.7°C (estimate)
- Density: 1.100
- Form: colorless tetragonal crystals
- Water Solubility: very soluble H2O [KIR78]
Reactions
Ammonium cyanide decomposes to ammonia and hydrogen cyanide, often forming a black polymer of hydrogen cyanide:
NH4CN → NH3 + HCN
It undergoes double decomposition reactions in solution with a number of metal salts.
It reacts with glyoxal, producing glycine (amino acetic acid):
NH4CN + (CHO)2 → NH2CH2COOH + HCN
Reactions with ketones yield aminonitriles, as in the first step of the Strecker amino acid synthesis:
NH4CN + CH3COCH3 → (CH3)2C(NH2)CN + H2O
When ammonium cyanate is heated for a short period of time, it produces urea, the first organic compound prepared in the laboratory. When urea is heated, a chemical compound that is soluble in hot water is formed.
Chemical analysis
Heat the salt and trap the decomposed products, hydrogen cyanide and ammonia, in water at low temperatures to analyze ammonium cyanide. The cyanide ion is measured in the aqueous solution using a silver nitrate titrimetric method or an ion-selective electrode method, and ammonia is measured using a titration or electrode technique.
Inorganic cyanides contain the cyanide group as the anion CN. Sodium cyanide and potassium cyanide are both extremely lethal soluble salts. Hydrocyanic acid, also known as hydrogen cyanide or HCN, is a highly volatile liquid that is widely produced in the industrial sector. It’s made from acidified cyanide salts.
Uses
In organic synthesis, ammonium cyanide is commonly used. Due to its instability, it is not shipped or sold commercially.
Toxicity
The solid or its solution is extremely poisonous. Ingestion can result in death. When exposed to the solid, it decomposes into highly toxic hydrogen cyanide and ammonia.