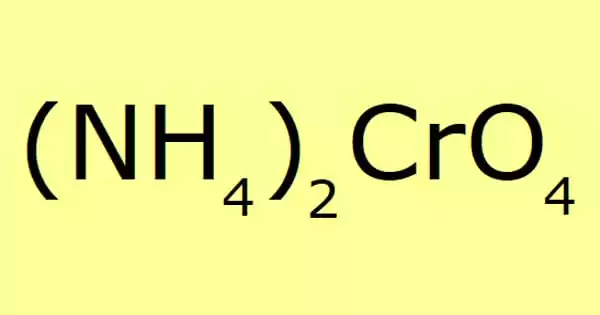Ammonium chromate is a salt having the formula (NH4)2CrO4. It is a yellow, monoclinic crystal formed by ammonium hydroxide and ammonium dichromate; it is employed in photography as a sensitizer for gelatin coatings. It appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride gas.
It is water-soluble and, when applied, can irritate mucous membranes, eyes, respiratory tract, skin, and other organs. After repeated contact, it may develop skin sensitization. It is also known to be carcinogenic (cancer-causing), and it can induce tissue ulceration as well as liver and kidney damage.
Properties
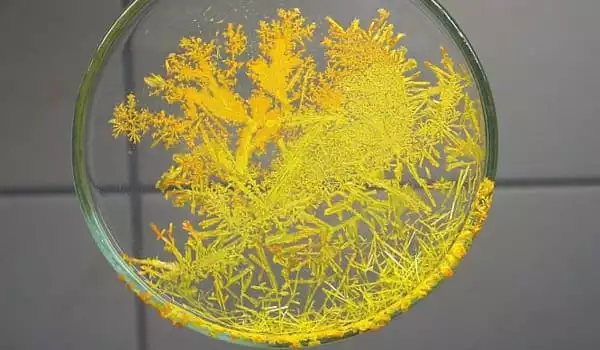
Ammonium chromate appears as a yellow crystalline solid. Its density is 1.866 g / cm3. It is soluble in water. It is toxic by inhalation (of dust). It is a strong irritant. The primary hazard is the threat to the environment.
- Molecular Weight: 152.07
- Appearance: Yellow-orange crystalline powder
- Melting Point: 180 °C
- Boiling Point: N/A
- Density: 1.91 g/cm3
- Solubility in H2O: N/A
Preparation Method
Neutralization method: Ammonium chromate solution is made by adding aqueous ammonia to a reactor containing ammonium dichromate to carry out the neutralization reaction, which is then followed by evaporation and concentration, cooling crystallization, solid-liquid separation, and drying to yield the final ammonium chromate product.
Double decomposition method: potassium chromate is added to a reactor containing water, and ammonium sulfate solution is slowly added under stirring to generate ammonium chromate and potassium sulfate solution, which is filtered to remove potassium sulfate and concentrated by evaporation, cooling crystallization, solid-liquid separation, drying, and prepared ammonium chromate products.
Application
It is used in dyeing, photography, chemical analysis, and as a corrosion inhibitor. It is often used in photography, textile printing, and fixing chromate dyes on wool. It is also used as an analytical reagent, catalyst, and corrosion inhibitor.
Reactivity Profile
It readily reacts with reducing materials, including large numbers of organic compounds. These reactions may be violent. An explosion hazard when shocked or heated. Emits toxic fumes of ammonia and nitrogen oxides when heated to decomposition.