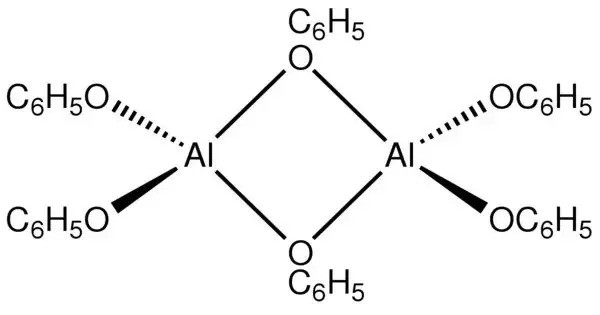
Aluminium phenolate, also called aluminium phenoxide, is an organometallic compound formed by the reaction of aluminium with phenol or its derivatives. It is the metalloorganic compound with the formula [Al(OC6H5)3]n. It can be represented generally as Al(OC₆H₅)₃, where aluminium is bonded to three phenoxide groups. It is a white solid. 27Al NMR studies suggest that aluminium phenolate exists in benzene solution as a mixture of dimer and trimer.
This compound belongs to the family of metal phenolates, in which the hydroxyl hydrogen of phenol is replaced by a metal atom. The compound can be prepared by the reaction of elemental aluminium with phenol:
Al + 3 HOC6H5 → Al(OC6H5)3 + 1.5 H2
The compound is used as a catalyst for the alkylation of phenols with various alkenes. For example, the ethylphenols are generated commercially by treating phenol with ethylene in the presence of a catalytic amount of aluminium phenolate.
Properties
It usually appears as a solid material that is sensitive to moisture and oxygen. In the presence of water, aluminium phenolate hydrolyzes easily, regenerating phenol and forming aluminium hydroxides. It is typically prepared by reacting aluminium chloride with sodium or potassium phenoxide, or by direct reaction of aluminium metal with phenol under controlled conditions.
- Chemical formula: C18H15AlO3
- Molar mass: 306.297 g·mol−1
- Appearance: white solid
It usually appears as a white to off-white powder or crystalline solid. The compound is sensitive to moisture and hydrolyzes easily, forming aluminium hydroxide and phenol. It is soluble in organic solvents like alcohols, ethers, and benzene derivatives, but insoluble in water due to rapid hydrolysis.
Chemically, aluminium phenolate acts as a strong Lewis base and exhibits good solubility in organic solvents. It is widely used as a catalyst in organic synthesis, especially in polymerization, esterification, and transesterification reactions. In the plastics industry, it plays a role in producing certain resins and stabilizing polymers. Furthermore, it serves as an intermediate in the preparation of other aluminium-organic compounds.
Occurrences
Aluminium phenolate does not occur naturally and is a purely synthetic compound. It is typically prepared through the reaction of aluminium metal, aluminium chloride, or aluminium alkoxides with phenol under controlled conditions. In industry and laboratory settings, it is mainly used as a reagent or catalyst, particularly in organic synthesis and polymer chemistry. Additionally, it has roles in producing phenolic resins, cross-linked polymers, and as an intermediate in coordination chemistry research.