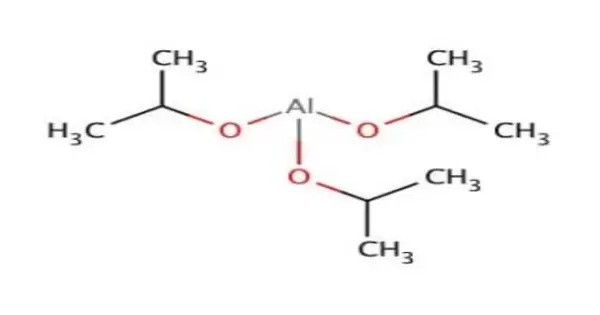
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis. It is a versatile organometallic compound widely used in organic synthesis and materials science.
Aluminium isopropoxide is moisture-sensitive, flammable, and requires storage in airtight containers under nitrogen. Its reactivity stems from the polarized Al-O bonds, making it an effective Lewis acid. While non-toxic, it can irritate skin and eyes, necessitating proper safety measures during handling. Its applications span pharmaceuticals, coatings, and nanotechnology, owing to its ability to form high-purity aluminium oxide.
Structure
A tetrameric structure of the crystalline material was verified by NMR spectroscopy and X-ray crystallography. The species is described by the formula Al[(μ-O-i-Pr)2Al(O-i-Pr)2]3. The unique central Al is octahedral, and three other Al centers adopt tetrahedral geometry. The idealised point group symmetry is D3.
Properties
It appears as a white crystalline solid or colorless liquid, highly reactive with water, forming aluminium hydroxide and isopropanol. Its molecular formula is C₉H₂₁AlO₃, with a molar mass of 204.24 g/mol. The compound is soluble in organic solvents like benzene and ethanol but hydrolyzes in moist air, requiring careful handling under inert conditions.
- Chemical formula: C9H21AlO3
- Molar mass: 204.246 g·mol−1
- Appearance: white solid
- Density: 1.035 g cm−3, solid
- Melting point: Sensitive to purity: 138–142 °C (99.99+%), 118 °C (98+%)
- Boiling point: @10 Torr 135 °C (408 K)
- Solubility in water: Decomposes
- Solubility in isopropanol: Poor
Chemical Properties
- Reactivity: Highly reactive with water and moisture, leading to hydrolysis. It must be handled under anhydrous conditions or in inert atmospheres (e.g., nitrogen or argon).
- Coordination Chemistry: Acts as a Lewis acid due to the aluminum center, forming coordination complexes with oxygen- or nitrogen-containing ligands.
- Stability: Sensitive to air and moisture; can decompose if not stored properly. Typically stored in sealed containers under inert conditions.
Preparation
This compound is commercially available. Industrially, it is prepared by the reaction between isopropyl alcohol and aluminium, or aluminium trichloride:
2 Al + 6 iPrOH → 2 Al(O-i-Pr)3 +3H2
AlCl3 + 3 iPrOH → Al(O-i-Pr)3 + 3 HCl
The procedure entails heating a mixture of aluminium, isopropyl alcohol, with a small amount of mercuric chloride. The process occurs via the formation of an amalgam of the aluminium. A catalytic amount of iodine is sometimes added to initiate the reaction. The industrial route does not use mercury.
Natural Occurrence
Aluminum isopropoxide does not occur naturally in the environment. It is a synthetic compound prepared in laboratory or industrial settings.
Synthesis
Typically synthesized by reacting aluminum metal or aluminum chloride with isopropanol in the presence of a catalyst (e.g., mercury(II) chloride) or under controlled conditions:
$$2Al + 6(CH₃)₂CHOH \rightarrow 2Al(OCH(CH₃)₂)₃ + 3H₂$$
The reaction requires anhydrous conditions to prevent hydrolysis.
Safety and Handling
- Hazards: Flammable solid; reacts vigorously with water, releasing flammable isopropanol vapors. Can cause skin and eye irritation.
- Storage: Store in airtight containers under inert gas (e.g., nitrogen) in a cool, dry place.
- Disposal: Must be hydrolyzed carefully under controlled conditions to avoid hazardous reactions.