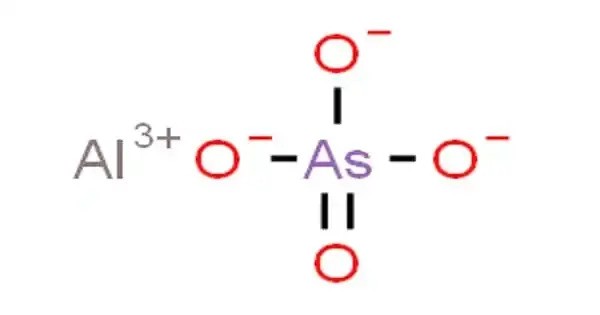
Aluminium arsenate is an inorganic compound with the formula AlAsO4. It has chemical and structural similarities with other metal arsenates and phosphates, and it occurs both naturally and synthetically. It is most commonly found as an octahydrate. It is a colourless solid that is produced by the reaction between sodium arsenate and a soluble aluminium salt. Aluminium arsenate occurs naturally as the mineral mansfieldite.
Modification of aluminium orthoarsenate was carried out by heating different samples to different temperatures. Both amorphous and crystalline forms were obtained. The solubility product was determined to be 10−18.06 for aluminium arsenate hydrate of formula AlAsO4·3.5H2O.
Properties
- Chemical formula: AlAsO4
- Molar mass: 165.899 g·mol−1
- Appearance: colourless crystals
- Density: 3.25 g/cm3
- Melting point: 1,000 °C (1,830 °F; 1,270 K)
- Solubility in water: insoluble
- Solubility product (Ksp): 10−18.06 for 2 AlAsO4.7 H2O
Chemical Structure
Aluminium arsenate crystallizes similarly to aluminium phosphate and can form different polymorphs:
- AlAsO₄·nH₂O: Hydrated forms exist, especially in natural settings.
- Anhydrous AlAsO₄: Often synthesized under controlled lab conditions.
Natural Occurrences
Aluminium arsenate occurs naturally as mineral species, mainly in oxidized zones of arsenic-bearing ore deposits. Notable Minerals:
- Mansfieldite (AlAsO₄·2H₂O) – the most common natural aluminium arsenate mineral.
- Occurs in weathered arsenic-rich environments.
- Typically found with other arsenates like scorodite (FeAsO₄·2H₂O).
Synthesis
Lab synthesis methods include:
- Precipitation method: Reacting aluminium salts (like Al₂(SO₄)₃) with sodium arsenate (Na₃AsO₄).
- Hydrothermal synthesis: Yields crystalline forms of AlAsO₄ with defined morphology.
Uses
Due to its toxicity (arsenic content), aluminium arsenate has limited commercial use, but it can serve roles in:
- Materials science: As a model compound for studying phosphate analogs.
- Environmental chemistry: Studying arsenic immobilization or remediation strategies.
Safety and Handling
- Toxic if inhaled or ingested due to arsenic content.
- Must be handled with gloves and respiratory protection in well-ventilated labs.
- Disposal requires hazardous waste protocols.