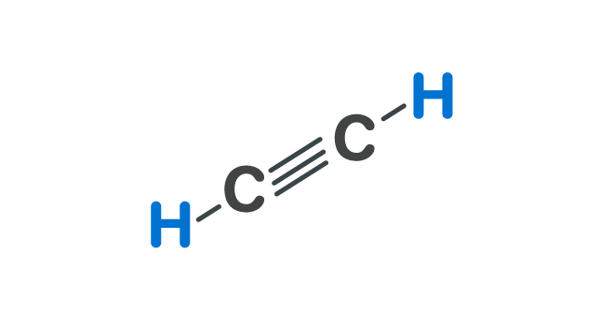
Acetylene appears as a colorless gas with a faint garlic-like odor. It is the chemical compound with the formula C2H2. It is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a hydrocarbon and the simplest alkyne. It is a colourless, inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. This colorless gas (lower hydrocarbons are generally gaseous in nature) is widely used as a fuel and a chemical building block.
It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is a colourless gas with a pleasant odour; as prepared from calcium carbide it usually contains traces of phosphine that cause an unpleasant garliclike odour. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities.
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. Acetylene is a terminal acetylenic compound, a gas molecular entity and an alkyne. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°.
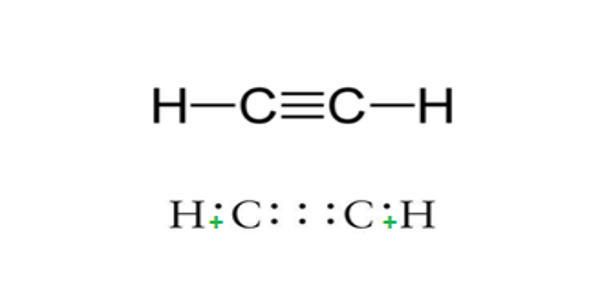
Physical properties
Acetylene is a non-toxic, colorless, and odorless gas. However, commercial grades contain impurities that lend a garlic-like odor to the gas. At atmospheric pressure, acetylene cannot exist as a liquid and does not have a melting point. The triple point on the phase diagram corresponds to the melting point (−80.8 °C) at the minimal pressure at which liquid acetylene can exist (1.27 atm). At temperatures below the triple point, solid acetylene can change directly to the vapour (gas) by sublimation. The sublimation point at atmospheric pressure is −84.0 °C.
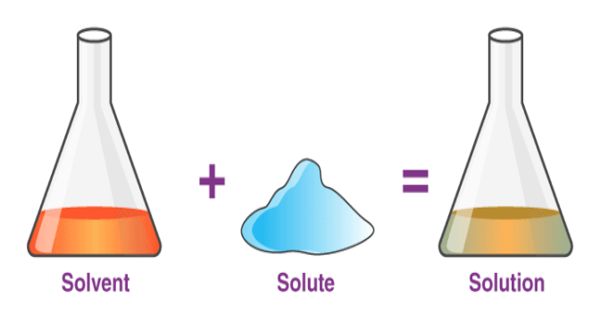
At room temperature, the solubility of acetylene in acetone is 27.9 g per kg. For the same amount of dimethylformamide (DMF), the solubility is 51 g. Acetylene gas is dissolved in dimethylformamide (DMF) or acetone, and supplied in special maroon-colored cylinders. At 20.26 bar, the solubility increases to 689.0 and 628.0 g for acetone and DMF, respectively. These solvents are used in pressurized gas cylinders.
Uses
- It is widely used as a chemical building block and as a fuel.
- It is used brazing.
- Used in the glass industry.
- Used in the manufacturing of synthetic rubber.
- Used as an additive to preserve food.
- Used to precipitate metals.
- Used in carburization of steel.
- Used as a fuel additive.
Hazards
Acetylene is a reactive material that poses a fire and explosion hazard. It is released to the environment through various industrial waste streams of industries. People who come in contact with this compound may suffer from headache, loss of consciousness, and dizziness. Its lower and upper explosive limits in air are 2.5% and 93%, respectively. Death due to choking can occur if a higher percentage of Ethyne is present in the air. Acetylene reacts with active metals (e.g., copper, silver, and mercury) to form explosive acetylide compounds.
Information Source: