About Citric Acid
Definition
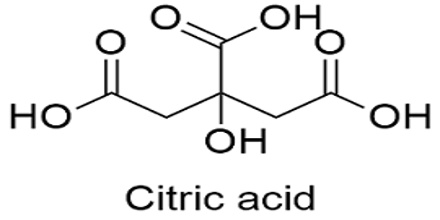
Citric acid is a white, odorless acid that has a sour taste and occurs widely in plants, especially in citrus fruit, and is formed during the Krebs cycle. Its chemical formula: C6H8O7. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. It is used in medicine and as a flavoring. Ions of citric acid are a by-product of the metabolism of carbohydrates during the Krebs cycle.
The acid gets its name from the citrus fruit family, which includes lemons, limes, oranges, tangerines, and grapefruits. Citrus fruits, especially the more sour ones like lemons and limes, owe their sharp taste to citric acid.
Production and Properties of Citric Acid
Citric acid is found naturally in citrus fruits, but producing citric acid from citrus fruits is very expensive and the demand for citric acid is greater than the available supply of citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices).
Citric acid from cane sugar can be kosher for Passover, wheat based citric acid is chometz, and the corn based citric acid is considered kitniyos shenishtanah, changed to a different entity during the process.
Citric acid was first isolated in 1784 by the chemist Carl Wilhelm Scheele, who crystallized it from lemon juice. It can exist either in an anhydrous (water-free) form or as a monohydrate. It is normally considered to be a tribasic acid, with pKa values, extrapolated to zero ionic strength, of 5.21, 4.28 and 2.92 at 25 °C.
Uses and Benefits of Citric Acid
Use citric acid on cut fresh fruit and when making homemade canned goods instead of lemon juice. The acid has the same effect as lemons in preventing fresh fruit from browning and regulates the pH level in canned goods to prevent bacteria growth. An easy use of citric acid is to use it in place of lemon juice in homemade lemonade! Dissolve one teaspoon of citric acid and sugar and adjust to taste in a glass of water.

Citric acid can be used as a substitute for salt in sour breads, such as sourdough and rye. Substitute an equal amount of citric acid for salt for the same flavor and none of the sodium.
Citric acid is an excellent chelating agent, binding metals by making them soluble. It is used to remove and discourage the buildup of limescale from boilers and evaporators. It can be used to treat water, which makes it useful in improving the effectiveness of soaps and laundry detergents. It can be used in shampoo to wash out wax and coloring from the hair.
Citric acid can be used as a lower-odor stop bath as part of the process for developing photographic film. Photographic developers are alkaline, so a mild acid is used to neutralize and stop their action quickly, but commonly used acetic acid leaves a strong vinegar odor in the darkroom.
Citric acid can help our bones absorb calcium more easily. Its ability to easily bond with minerals aids the absorption and digestion of minerals. Citric acid can also help prevent small kidney stones from growing into larger, problematic stones. The citric acid coats the stones and prevents materials from sticking to them and making them larger.
Reference: nuts.com, ok.org, dictionary.com, wikipedia.