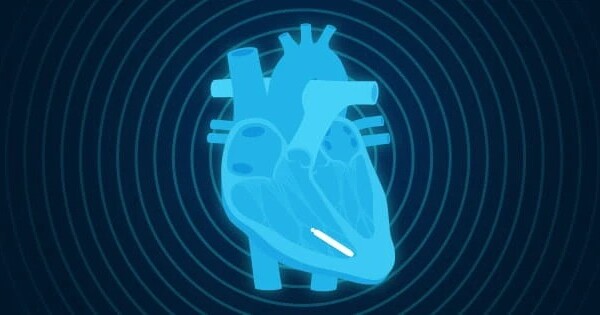
According to the findings of a multi-center, international clinical trial co-led by a Weill Cornell Medicine and novel York-Presbyterian investigator, a dual-chamber wireless pacemaker provides consistent performance across three months, adding to the evidence for this novel pacemaker alternative.
The findings, published in Circulation, revealed that the two microscopic components of this wireless pacemaker device communicate consistently, meeting the goal of coordinating contractions of the upper and lower heart chambers 98% of the time. The research team, which included Dr. James Ip, a clinical medicine professor at Weill Cornell Medicine and a cardiac electrophysiologist at NewYork-Presbyterian/Weill Cornell Medical Center, discovered that the device continued to function properly even when patients engaged in various activities or had their heart rates elevated.
“We’ve entered a new era of pacing,” Dr. Ip said. “We are not limited to the wired devices we’ve been using for more than 60 years. We now have new technology and methods to help patients and reduce complications.”
We have proven that the two separate pieces of the new pacemaker system communicate with each other very well even when people change positions, engage in activities, or when their heart rates rise. Also, the device actually performs better than how it tells you it is functioning.
Dr. Ip
Pacemakers deliver an electrical signal to regulate the heartbeat in people with slow heart rhythms. A traditional pacemaker is implanted under the skin in the chest and has wires (leads) threaded through veins to the heart. However, the devices can lead to potential complications.
“The wire is the Achilles heel of the pacemaker,” Dr. Ip said. “If you bend it enough times, it can break. While wired pacemakers have been the standard-of-care for reliable pacing for years, the technology can be improved.”
If the wire breaks, doctors must remove it, which might be difficult if considerable scar tissue has grown around it over time. Bacteria may also cling to the wire, causing potentially severe bloodstream infections. People who engage in activities that require a lot of arm movement may be more susceptible to wires breaking. Individuals who are thinner or have thinner skin are at danger of having the device erode through the skin. Patients with blocked vessels, weakened immune systems, dialysis, or an increased risk of infection may not be suitable candidates for wired pacemakers.
About ten years ago, new wireless pacemakers became available that can stimulate the heart’s lower chamber, called the ventricle, providing an alternative to wired pacemakers for patients with some types of heart rhythm problems. The tiny devices are threaded through a blood vessel in the groin to the heart and screwed in place, a procedure that is less invasive than the implantation of wired devices.

In May 2023, a clinical trial published in the New England Journal of Medicine, co-authored by Dr. Ip, demonstrated the safety and effectiveness of a wireless pacemaker implanted in the heart’s upper chamber, the atrium, that can stimulate both the upper and lower chambers. The study took place at 55 sites across the United States, Canada, and Europe, including NewYork-Presbyterian/Weill Cornell Medical Center and NewYork-Presbyterian/Columbia University Irving Medical Center. In July 2023, the U.S. Food and Drug Administration (FDA) approved the device based on the study’s results, making the option of a wireless pacemaker available to patients with a broader range of heart rhythm problems.
The present study expands on earlier findings by giving longer-term data on how consistently the dual chamber stimulating device functions in a variety of situations, such as when patients participate in activities that raises their heart rate or change positions, such as standing or lying down. During a three-month follow-up visit, study investigators requested 384 individuals who had participated in the first clinical trial to wear an external heart monitor to evaluate the device’s functionality while performing various activities.
“We have proven that the two separate pieces of the new pacemaker system communicate with each other very well even when people change positions, engage in activities, or when their heart rates rise,” Dr. Ip said. “Also, the device actually performs better than how it tells you it is functioning.”
As a result, Dr. Ip believes practitioners should be able to depend on the devices’ recordings rather than requiring patients to wear a cumbersome cardiac monitor to check their performance. He emphasized that the devices are still first-generation and have some limitations. For example, their little batteries may not last as long as those used in typical wired pacemakers. However, he believes that as technology advances, leadless pacemakers will improve even more. Meanwhile, he and his colleagues will continue to analyze the devices’ function, stating that they provide patients with a valuable choice.
“I’ve been involved in researching and implanting wireless pacemakers for over a decade, starting with animal studies, then clinical trials in humans, and now after FDA approval,” Dr. Ip said. “It is very gratifying to see how the technology has evolved over time and how these devices genuinely help people.”