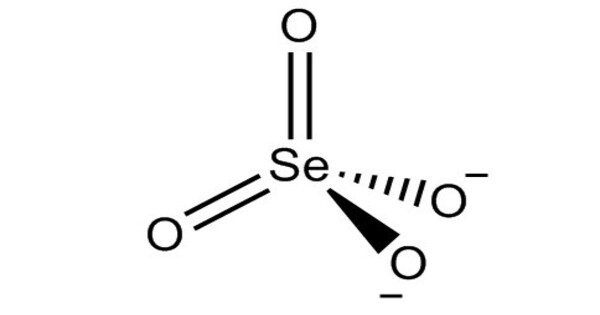
Zirconium selenate is an inorganic compound with the chemical formula Zr(SeO4)2. It is a chemical compound consisting of zirconium and selenate ions. Its tetrahydrate can be obtained by the reaction of selenic acid and a saturated aqueous solution of zirconium oxychloride octahydrate (or zirconium hydroxide).
The tetrahydrate belongs to the orthorhombic crystal system and is isostructural with Zr(SO4)2·4H2O. It loses water when heated and becomes anhydrous at 220-230 °C. It reacts with potassium fluoride to obtain K2Zr(SeO4)2F2·3H2O.
Properties
Zirconium selenate is a crystalline solid. It is generally soluble in water. It is stable under standard conditions but can decompose at higher temperatures. Like many selenates, it can be toxic and should be handled with care. Proper safety equipment such as gloves and eye protection should be used.
- Chemical formula: Zr(SeO4)2
- Appearance: colourless crystals (tetrahydrate)
- Density: 3.806 g·cm−3 (monohydrate), 3.36 g·cm−3 (tetrahydrate)
- Boiling point: 580 °C (dec.)
- Solubility in water: soluble (tetrahydrate)
Applications
- Catalysis: Used in certain catalytic processes due to the unique properties of zirconium.
- Materials Science: Incorporated into various materials for its chemical and physical properties.
Safety and Handling
- Toxicity: As with many selenates, zirconium selenate should be handled with care due to potential toxicity. Inhalation, ingestion, or skin contact should be avoided.
- Storage: Should be stored in a cool, dry place, away from incompatible materials.