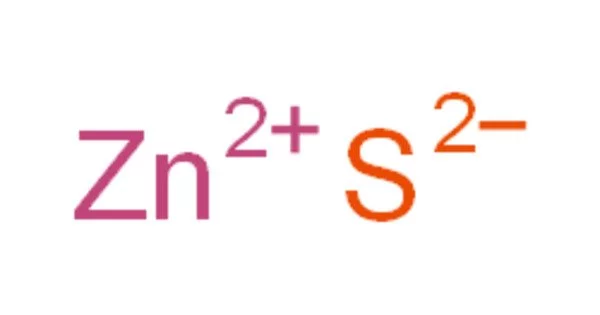Zinc sulfide, also known as ZnS, is an inorganic compound. It is a naturally occurring salt that serves as the primary source of zinc. This is the most common form of zinc found in nature, where it is mostly found in the mineral sphalerite. Although it is usually black due to impurities, the pure material is white and is widely used as a pigment. Zinc sulfide, in its dense synthetic form, can be transparent and is used as a window for visible and infrared optics.
Zinc sulfide is most commonly used as a pigment. Lithopone, when combined with barium sulfate, produces a low gloss interior house paint.
Properties
Zinc sulfide appears as a yellowish-white powder in a liquid. It is insoluble in water and denser than water. It occurs in nature in two crystalline forms, the minerals, wurtzite, and sphalerite. Sulfide ore is the principal zinc mineral.
- Chemical formula: ZnS
- Molar mass: 97.474 g/mol
- Density: 4.090 g/cm3
- Melting point: 1,850 °C (3,360 °F; 2,120 K) (sublime)
- Solubility in water: negligible

Structure
ZnS exists in two major crystalline forms, and this dichotomy is a prominent example of polymorphism. The coordination geometry at Zn and S is tetrahedral in each form. Zinc blende or sphalerite are other names for the more stable cubic form. The hexagonal form is known as wurtzite, a mineral that can also be produced synthetically. The transition temperature from sphalerite to wurtzite is around 1020 °C. A tetragonal form is also known as polhemusite, a very rare mineral with the formula (Zn,Hg)S.
Production
Zinc sulfide is extracted from natural deposits and concentrated using various methods. In the laboratory, zinc sulfide can also be made by passing hydrogen sulfide through an aqueous solution of a soluble zinc salt, such as zinc chloride or zinc nitrate. Filtered, washed, and dried precipitate
Zinc sulfide is typically made from byproducts of other processes. Smelter, slag, and pickle liquors are common sources. It is also a byproduct of the ammonia synthesis from methane, where zinc oxide is used to remove hydrogen sulfide impurities from natural gas:
ZnO + H2S → ZnS + H2O
Laboratory preparation
It is easily produced by igniting a mixture of zinc and sulfur. Since zinc sulfide is insoluble in water, it can also be produced in a precipitation reaction. Solutions containing Zn2+ salts readily form a precipitate ZnS in the presence of sulfide ions (e.g., from H2S).
Zn2+ + S2− → ZnS
This reaction is the basis of a gravimetric analysis for zinc.
Applications
- Luminescent material
Zinc sulfide, with addition of few ppm of suitable activator, exhibits strong phosphorescence (described by Nikola Tesla in 1893), and is currently used in many applications, from cathode ray tubes through X-ray screens to glow in the dark products.
- Optical material
Zinc sulfide is also used as an infrared optical material, transmitting from visible wavelengths to just over 12 micrometers. It can be used planar as an optical window or shaped into a lens.
- Pigment
Zinc sulfide is a common pigment, sometimes called sachtolith. When combined with barium sulfate, zinc sulfide forms lithopone.
- Catalyst
When illuminated, fine ZnS powder acts as an efficient photocatalyst, producing hydrogen gas from water. During the synthesis of ZnS, sulfur vacancies can be introduced, gradually transforming the white-yellowish ZnS into a brown powder and increasing photocatalytic activity through increased light absorption.
- Semiconductor properties
Both sphalerite and wurtzite are intrinsic semiconductors with a wide bandgap. These are typical II-VI semiconductors with structures similar to many other semiconductors, such as gallium arsenide.