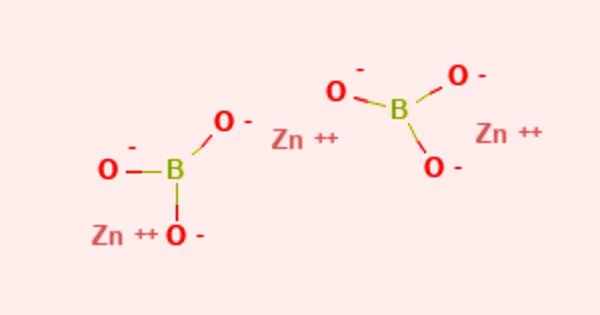
Zinc borate is an inorganic compound that is a zinc borate. It appears as a white powder with varying chemical composition. It is insoluble in water white crystalline or amorphous powder. It is only marginally soluble in water. The main risk is the threat to the environment. It has low toxicity. It has a melting point of 980 °C.
Zinc Borate is boron-based flame retardant that is compatible with a wide range of polymeric matrices. It is effective in both the solid and gas phases, and its strong smoke suppressing action helps to improve rescue time in the event of a fire. It is used as a fungus and mildew inhibitor, as well as to fireproof textiles. Immediate action should be taken to limit its environmental spread.
Properties
Zinc borate inhibits afterglow and acts as an anti-dripping and char-promoting agent. It prevents arcing and tracking in electrical insulator plastics. As a corrosion inhibitor pigment, zinc borate works in tandem with zinc phosphate or barium borate. In plastics and wood products, it acts as a broad-spectrum fungicide.
- Compound Formula: B2O6Zn3
- Molecular Weight: 313.7584 g/mol
- Appearance: White solid
- Melting Point: 980 °C
- Boiling Point: N/A
- Density: 3.64 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 311.772416 g/mol

Uses
Zinc borate is primarily used in plastics and cellulose fibers, paper, rubbers, and textiles as a flame retardant. It’s also found in paints, glues, and pigments. As a flame retardant, it can act as a synergist with antimony(III) oxide in both halogen-based and halogen-free systems. It prevents dripping and promotes char, as well as suppressing afterglow. It prevents arcing and tracking in electrical insulator plastics.
Zinc borate is used in halogen-containing systems alongside antimony trioxide and alumina trihydrate. It catalyzes the formation of char and forms a glass protective layer. Zinc catalyzes halogen release by forming zinc halides and zinc oxyhalides.
Zinc borate can be used in halogen-free systems with alumina trihydrate, magnesium hydroxide, red phosphorus, or ammonium polyphosphate. When the plastics are burned, a porous borate ceramics is formed, which protects the underlying layers. Borosilicate glass can be formed at plastic burning temperatures in the presence of silica.
Polyvinyl chloride, polyolefins, polyamides, epoxy resins, polyesters, thermoplastic elastomers, rubbers, and other materials contain zinc borate. It’s also used in some fire suppression systems. In some ceramics, it can be used as a flux. It improves the properties of ceramics in electrical insulators. Nanopowder zinc borate can be used for the aforementioned applications as well as to improve the frictional properties of lubricating oils.
Zinc borates have numerous applications. It is ideal for polymers and polyamides due to its flame retardant and smoke suppression properties. For the same reasons, it is used in paper, rubber, and textiles. Zinc borate is used in agriculture as plant nutrition. It is also used as a fungicide to extend the life of wood. It’s useful in cables and insulators, as well as as an arc suppressant. It is even used to improve the friction properties of lubricants. This versatile mineral has applications in nearly every industry.