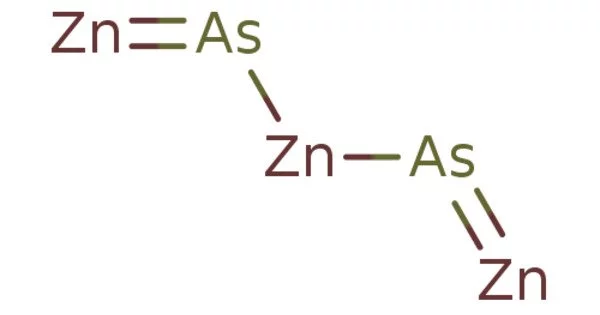Zinc arsenide (Zn3As2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is a chemical compound of zinc and arsenic. Zinc is a metallic element with the atomic number 30. It is found in nature most often as the mineral sphalerite. It is an inorganic semiconductor with a band gap of 1.0 eV.
Zinc arsenide is often used as a component in the manufacture of semiconductors.
Properties
- Chemical formula: Zn3As2
- Molar mass: 345.984 g/mol
- Appearance: Silver grey
- Density: 5.53 g/cm3
- Melting point: 1,015 °C (1,859 °F; 1,288 K)
- Solubility in water: Insoluble
- Crystal structure: Tetragonal

Synthesis and reactions
Zinc arsenide can be prepared by the reaction of zinc with arsenic
3 Zn + 2 As → Zn3As2
Structure
Zn3As2 has a tetragonal form at room temperature that converts to a different tetragonal phase at 190 °C and a third phase at 651 °C. The zinc atoms are tetrahedrally coordinated in the room-temperature form, and the arsenic atoms are surrounded by six zinc atoms at the vertices of a distorted cube. Zinc arsenide has a crystalline structure that is very similar to cadmium arsenide (Cd3As2), zinc phosphide (Zn3P2), and cadmium phosphide (Cd3P2). These Zn-Cd-P-As quaternary system compounds exhibit full continuous solid-solution.
Arsenic is found free in nature in three metalloidal forms with different crystal structures (the minerals arsenopyrite and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as a compound with other elements.
Electronic structure
Its lowest direct and indirect bandgaps are within 30 meV or each other.
Safety
Though excessive zinc is harmful, it is necessary for life in small amounts because it is a cofactor for over 300 enzymes and is found in nearly as many transcription factors. Arsenic is a chemical element with the atomic number 33 and the symbol As. It is a poisonous metalloid with many allotropic forms, including yellow (molecular non-metallic) and several black and grey forms (metalloids).