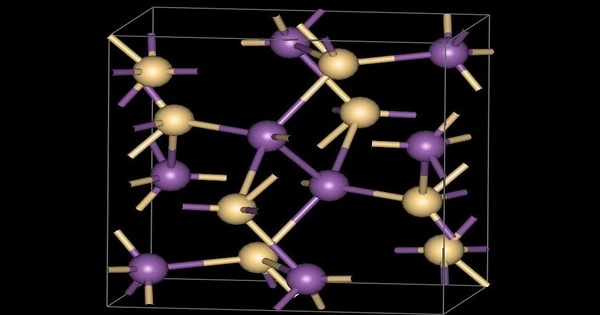
Zinc antimonide is a chemical compound that is inorganic. It has the chemical formula ZnSb and is a gray solid. Six intermetallics are present in the Zn-Sb system. It is made up of zinc and antimonide ions. It is a semiconducting intermetallic compound, similar to indium antimonide, aluminum antimonide, and gallium antimonide. It can be found in transistors, infrared detectors, thermal imagers, and magnetoresistive devices.
Properties
Its properties are between that of alloy and salt. It is made up of zinc and antimonide ions. Zinc antimonide reacts with water to form stibine. It acts as a reducing agent, and is prepared by heating zinc and antimony.
- Compound Formula: Zn4Sb3
- Molecular Weight: 626.84
- Appearance: Solid in various forms (powder, pieces, disc, sputtering target)
- Melting Point: 570 °C
- Boiling Point: N/A
- Density: 6.33 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 626.421007
- Crystal Structure: Orthorhombic

The first reported use of zinc-antimony alloys was in the original work of T. J. Seebeck on thermoelectricity, a scientist who would later give his name to the Seebeck effect. By the 1860s, Moses G. Farmer, an American inventor, had created the first high-powered thermoelectric generator based on a zinc-antimony alloy with a composition very close to stoichiometric ZnSb. He displayed this generator at the 1867 Paris Exposition, where it was carefully studied and copied (with minor modifications) by a number of people, including Clamond.
In 1870, Farmer was granted a patent for his generator. In the early 1900s, George H. Cove patented a thermoelectric generator based on a Zn-Sb alloy. His patent claimed a voltage and current of 3V at 3A for six “joints.” This was a far higher output than would be expected from a thermoelectric couple, and it was possibly the first demonstration of the thermophotovoltaic effect, as the bandgap for ZnSb is 0.56eV, which could yield close to 0.5V per diode under ideal conditions. Mária Telkes, who worked at Westinghouse in Pittsburgh in the 1930s, was the next researcher to work with the material. The discovery of the higher bandgap Zn4Sb3 material in the 1990s rekindled interest.
Zinc antimonide finds applications in the following:
The zinc antimonide ZnSb is one of the most materials which have been studied intensively in recent years, due to its importance in thermoelectric applications, and as well as its use as an electrode in rechargeable Li-ion batteries.
- Transistors
- Thermal imagers
- Infrared detectors
- Magnetoresistive devices