A useful natural system for storing carbon dioxide is photosynthesis. Simply producing biomass, though, does not fully utilize this mechanism. A microbial community that may act as a living carbon sink has been genetically produced by a Chinese research team and published in the journal Angewandte Chemie.
In this community, photosynthesis first turns carbon dioxide into sugar, which is later transformed into valuable compounds. In biotechnology, different bacterial strains are employed to create particular compounds.
For example, some genetically modified strains produce lactic acid, which in turn is used to produce the biodegradable plastic, polylactic acid (PLA). Other strains are employed to enrich biofuel or medicinal precursors. However, bacterial synthesis of compounds is frequently ineffective since the bacteria need energy and nutrition.
In contrast, phototrophic organisms rely on carbon dioxide, water, and sunlight to spontaneously generate sugar. Therefore, in a symbiotic community, chemical-producing bacteria might hypothetically consume this sugar as food, making them a possible carbon sink while produce useful compounds at the same time.
However, a lot of photoautotrophs create sucrose as their stored sugar, which bioengineered bacteria find difficult to use and consume.
To find prospective bacterial strains that could be bioengineered but could also grow naturally on sucrose, the research team of Jun Ni at Shanghai Jiao Tong University in Shanghai (China) conducted a methodical search. They found what they were looking for in a marine bacterium known as Vibrio natriegens: “Luckily, V. natriegens naturally harbors the complete sucrose transport and metabolism pathway,” reveal the authors.
In addition, V. natriegens can be genetically manipulated and tolerates salt stress. This is significant because salt induces the production of sucrose by photosynthetic cyanobacteria, resulting in a feedback loop of activities.
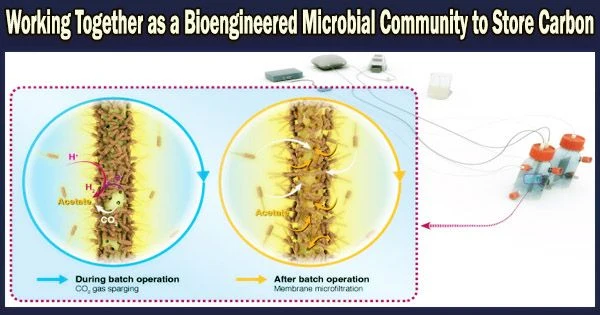
Using this information, the study team created an integrated modular system for CO2 sequestration from V. natriegens and the well-known cyanobacterium Synechococcus elongatus. They used genetic engineering to increase sugar production in the cyanobacteria and genetically modified V. natriegens to increase sugar intake and chemical conversion.
The team discovered that the cyanobacteria may bundle the nutrients in vesicles that are later expelled, a procedure that was surprisingly effective. These vesicles might then be easily consumed by the marine bacteria.
In order to produce lactic acid, butanediol for the manufacture of biofuel, coumarin and melanin as precursors for chemicals and pharmaceuticals, or butanediol and melanin respectively, the researchers created four varieties of V. natriegens. The cyanobacteria and the bacteria worked together in symbiosis to manufacture the compounds with a negative carbon balance.
“This system could absorb more than 20 tons of carbon dioxide per ton of product,” the team report. The authors consider their results to be proof that symbiotic microbial communities can be used as effective carbon sinks.