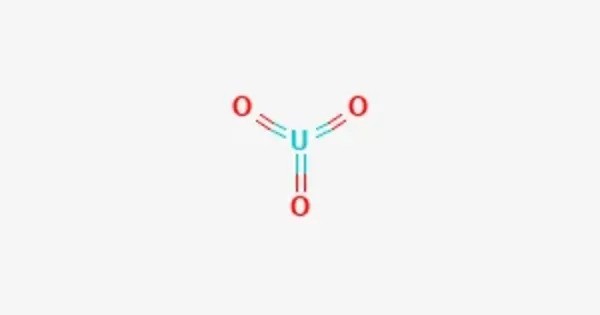
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. It is an inorganic compound composed of uranium and oxygen, commonly found as a yellow to orange crystalline solid. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph is amorphous UO3. It is one of the most important oxides of uranium and serves as an intermediate in the conversion of uranium ores into nuclear fuel.
Uranium trioxide typically forms when uranium dioxide (UO₂) or uranyl nitrate is heated in air, producing a compound in which uranium is in the +6 oxidation state. Chemically, UO₃ is amphoteric, capable of reacting with acids to form uranyl salts (such as uranyl nitrate) and with bases to produce uranates. It is only slightly soluble in water but readily dissolves in acids. Uranium trioxide exists in several polymorphic forms depending on temperature and humidity, including α-, β-, and γ-UO₃.
Properties
Uranium trioxide (UO₃) is an oxide of uranium, typically appearing as an orange or yellow crystalline solid. It has a molecular weight of 286.03 g/mol and a density of about 7.3 g/cm³. UO₃ is the highest oxide of uranium and acts as an intermediate compound between uranium dioxide (UO₂) and uranium tetrafluoride (UF₄) in the nuclear fuel cycle. It is amphoteric and can react with acids to form uranyl salts. When heated, UO₃ can decompose to uranium dioxide and oxygen. It is radioactive, insoluble in water, and slightly soluble in acids and alkalis.
Reactivity
Uranium trioxide reacts at 400 °C with freon-12 to form chlorine, phosgene, carbon dioxide and uranium tetrafluoride. The freon-12 can be replaced with freon-11 which forms carbon tetrachloride instead of carbon dioxide. This is a case of a hard perhalogenated freon which is normally considered to be inert being converted chemically at a moderate temperature.
2 CF2Cl2 + UO3 → UF4 + CO2 + COCl2 + Cl2
4 CFCl3 + UO3 → UF4 + 3 COCl2 + CCl4 + Cl2
Uranium trioxide can be dissolved in a mixture of tributyl phosphate and thenoyltrifluoroacetone in supercritical carbon dioxide, ultrasound was employed during the dissolution.
In the nuclear industry, UO₃ is a precursor to uranium dioxide fuel and uranium hexafluoride (UF₆), which is used in uranium enrichment processes. It is also encountered as a product of oxidation of spent uranium fuel. Due to its radioactivity and chemical toxicity, uranium trioxide must be handled with great care under controlled conditions, adhering to strict safety and environmental regulations.
Occurrences
Uranium trioxide is not found naturally in pure form but can occur as a secondary oxidation product of uranium minerals such as uraninite and pitchblende. It is commonly produced industrially during the refining and conversion of uranium ores. UO₃ also appears in the processing of yellowcake (U₃O₈) into uranium hexafluoride (UF₆) for nuclear fuel fabrication.
Health and safety hazards
Like all hexavalent uranium compounds, UO3 is hazardous by inhalation, ingestion, and through skin contact. It is a poisonous, slightly radioactive substance, which may cause shortness of breath, coughing, acute arterial lesions, and changes in the chromosomes of white blood cells and gonads leading to congenital malformations if inhaled. However, once ingested, uranium is mainly toxic for the kidneys and may severely affect their function.